
Which of the following alcohol reacts with ${\text{HBr}}$ at the fastest rate?
A.
B.
C.
D.




Answer
578.1k+ views
Hint: To solve this question, you must recall the mechanism of nucleophilic substitution. In an ${{\text{S}}_{\text{N}}}{\text{2}}$ mechanism, the incoming nucleophile attacks the carbon atom from backside and results in inversion in configuration. While in an ${{\text{S}}_{\text{N}}}1$ mechanism, a carbocation intermediate is formed, which on attack by the nucleophile gives the product.
Complete step by step answer:
In a bimolecular nucleophilic substitution reaction, since the attack of nucleophile and the departure of leaving group take place at the same time, attack from the front side by incoming nucleophile is hindered due to the leaving nucleophile. Thus, the nucleophile attacks the carbon from the back side.
In this case, the parent alkyl part is a cyclohexane ring. Thus, a backside attack on the carbon atom is hindered. As a result, we can say that the reaction proceeds through the unimolecular nucleophilic substitution reaction (${{\text{S}}_{\text{N}}}1$).
It is a first order reaction. The rate determining step of the reaction involves only the carbocation intermediate. In the first step, ${{\text{H}}^{\text{ + }}}$ attacks on the hydroxyl group which then departs as a water molecule leaving behind a carbocation.
Thus, that alcohol reacts with ${\text{HBr}}$ at the fastest rate which forms the most stable carbocation. We know that tertiary carbocation is most stable due to the electron donating inductive effect and hyperconjugation of three carbon atoms.
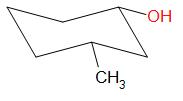
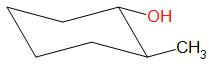
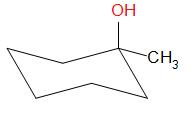
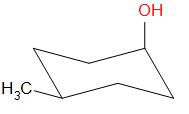
Options A, B and D result in the formation of secondary carbocations while in option C, a tertiary carbocation intermediate is formed.
Thus, it reacts at the fastest rate.
The correct option is C.
Note:
The rates of reactivity of alcohols towards ${{\text{S}}_{\text{N}}}1$ reaction mechanism are as follows:
Tertiary alcohol > Secondary alcohol > Primary alcohol
Primary alcohols do not undergo ${{\text{S}}_{\text{N}}}1$ reaction as primary carbocation is not stable.
Complete step by step answer:
In a bimolecular nucleophilic substitution reaction, since the attack of nucleophile and the departure of leaving group take place at the same time, attack from the front side by incoming nucleophile is hindered due to the leaving nucleophile. Thus, the nucleophile attacks the carbon from the back side.
In this case, the parent alkyl part is a cyclohexane ring. Thus, a backside attack on the carbon atom is hindered. As a result, we can say that the reaction proceeds through the unimolecular nucleophilic substitution reaction (${{\text{S}}_{\text{N}}}1$).
It is a first order reaction. The rate determining step of the reaction involves only the carbocation intermediate. In the first step, ${{\text{H}}^{\text{ + }}}$ attacks on the hydroxyl group which then departs as a water molecule leaving behind a carbocation.
Thus, that alcohol reacts with ${\text{HBr}}$ at the fastest rate which forms the most stable carbocation. We know that tertiary carbocation is most stable due to the electron donating inductive effect and hyperconjugation of three carbon atoms.
Options A, B and D result in the formation of secondary carbocations while in option C, a tertiary carbocation intermediate is formed.
Thus, it reacts at the fastest rate.
The correct option is C.
Note:
The rates of reactivity of alcohols towards ${{\text{S}}_{\text{N}}}1$ reaction mechanism are as follows:
Tertiary alcohol > Secondary alcohol > Primary alcohol
Primary alcohols do not undergo ${{\text{S}}_{\text{N}}}1$ reaction as primary carbocation is not stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




