
Which of the following acids on decarboxylation gives Isobutane?
A)2,2-Dimethyl butanoic acid
B)2,2-dimethyl propanoic acid
C)3-Methyl pentanoic acid
D)2-Methyl butanoic acid
Answer
489k+ views
Hint: Decarboxylation is the removal of a carboxyl group from the compound which results in the formation of an alkane and carbon dioxide. If the given acid has no branches, then the resultant alkane will be a straight chain and if the acid has one or two branches of methyl group it will form a branched-chain alkane. The resultant alkane can be secondary or tertiary alkane.
Complete Step By Step Answer:
Here, we have to find a compound that undergoes decarboxylation to form an Isobutane. Isobutane is a secondary alkane.
Usually, decarboxylation of the alkyl acid is the reaction for the preparation of Isobutane.
Now we have to find the compound which will lose a carboxyl group to form Isobutane.
The carbon and oxygen in the carboxyl group are preferred from the carboxyl group of the compound. The carbon leaving the compound is from the –COOH of the parent compound, which is the first carbon chain.
To form an Isobutane, the compound to undergo decarboxylation should have only 5 carbon atoms. Since 4 carbon atoms will form the Isobutane and one carbon will leave as carbon-di-oxide.
Now, Option A: 2,2-Dimethyl butanoic acid and Option C: 3-Methyl pentanoic acid will be discarded because they have 6 carbons in the compound.
So, we are left with Option B and Option D.
Let’s have a look at the decarboxylation of 2,2-dimethyl propanoic acid in the presence of NaOH and CaO.
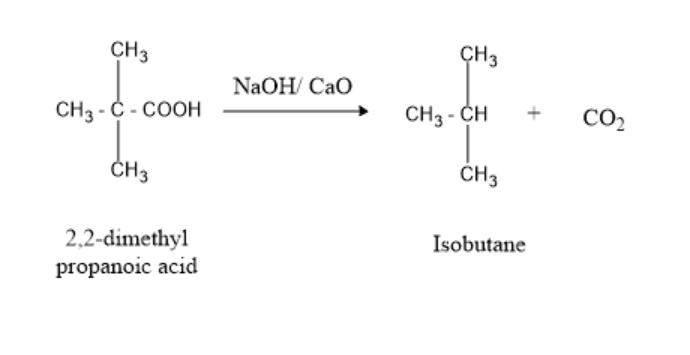
The decarboxylation of 2,2-dimethyl propanoic acid in the presence of NaOH and CaO forms Isobutane and carbon-di-oxide.
Similarly, the decarboxylation of 2-Methyl butanoic acid forms n-butane.
Therefore, 2,2-dimethyl propanoic acid forms Isobutane.
Final answer: The correct answer is Option B: 2,2-dimethyl propanoic acid.
Note:
Isobutane can also be formed by the decarboxylation of 3-methylbutanoic acid. Also, n-butane can be formed by Wurtz synthesis of an Ethyl bromide or hydrolysis of n-butyl magnesium bromide. However, reduction of alcohol results in the formation of the same parent compound.
Complete Step By Step Answer:
Here, we have to find a compound that undergoes decarboxylation to form an Isobutane. Isobutane is a secondary alkane.
Usually, decarboxylation of the alkyl acid is the reaction for the preparation of Isobutane.
Now we have to find the compound which will lose a carboxyl group to form Isobutane.
The carbon and oxygen in the carboxyl group are preferred from the carboxyl group of the compound. The carbon leaving the compound is from the –COOH of the parent compound, which is the first carbon chain.
To form an Isobutane, the compound to undergo decarboxylation should have only 5 carbon atoms. Since 4 carbon atoms will form the Isobutane and one carbon will leave as carbon-di-oxide.
Now, Option A: 2,2-Dimethyl butanoic acid and Option C: 3-Methyl pentanoic acid will be discarded because they have 6 carbons in the compound.
So, we are left with Option B and Option D.
Let’s have a look at the decarboxylation of 2,2-dimethyl propanoic acid in the presence of NaOH and CaO.

The decarboxylation of 2,2-dimethyl propanoic acid in the presence of NaOH and CaO forms Isobutane and carbon-di-oxide.
Similarly, the decarboxylation of 2-Methyl butanoic acid forms n-butane.
Therefore, 2,2-dimethyl propanoic acid forms Isobutane.
Final answer: The correct answer is Option B: 2,2-dimethyl propanoic acid.
Note:
Isobutane can also be formed by the decarboxylation of 3-methylbutanoic acid. Also, n-butane can be formed by Wurtz synthesis of an Ethyl bromide or hydrolysis of n-butyl magnesium bromide. However, reduction of alcohol results in the formation of the same parent compound.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




