
Which molecule is the only electron donor?
A.${\text{N}}{{\text{H}}_{\text{3}}}$
B.${\text{B}}{{\text{F}}_{\text{3}}}$
C.\[{\text{P}}{{\text{F}}_{\text{3}}}\]
D.${\text{As}}{{\text{F}}_{\text{3}}}$
Answer
559.2k+ views
Hint: We should know the condition which makes a molecule electron donor. Simply a molecule to be an electron donor should have excess or free electrons density. The electron density can be in the form of lone pair or negative charge. As we have to find out only electron donors, we will check if the electron density is available or not at a molecule and we all check that is there any factor making the molecule electron acceptor.
Complete answer:
A molecule that donates electrons is known as electron donor and the molecule which accepts electrons is known as electron acceptor.

${\text{N}}{{\text{H}}_{\text{3}}}$ known as ammonia has one lone pair on a nitrogen atom.
${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{3}}}$
The electronic configuration of nitrogen is . So, nitrogen has three electrons in p-orbital, so it accepts three electrons from three hydrogens and forms an ammonia molecule. Now there is no vacant p-orbital present in nitrogen so, ammonia molecule is only an electron donor because it can donate its lone pair but cannot accept electrons.
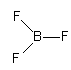
${\text{B}}{{\text{F}}_{\text{3}}}$, The electronic configuration of boron is ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^1}$. So, boron has only one electron in p-orbital so, it accept three electrons from three fluorine atoms and form ${\text{B}}{{\text{F}}_{\text{3}}}$molecule. ${\text{B}}{{\text{F}}_{\text{3}}}$molecule does not has any lone pair or negative charge, so it cannot work as an electron donor.
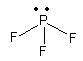
\[{\text{P}}{{\text{F}}_{\text{3}}}\], The electronic configuration of phosphorus is ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{3}}}{\text{3d}}$. So, phosphorus has one three electrons in p-orbital so, it accepts three electrons from three fluorine atoms and \[{\text{P}}{{\text{F}}_{\text{3}}}\] molecule. Now \[{\text{P}}{{\text{F}}_{\text{3}}}\]molecule has one lone pair so, it can work as electron donor but it also has vacant d-orbital so, it also accept electrons so, it is not only electron donor.
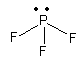
${\text{As}}{{\text{F}}_{\text{3}}}$, The electronic configuration of arsenic is ${\text{[Ar]3}}{{\text{d}}^{10}}{\text{4}}{{\text{s}}^2}{\text{4}}{{\text{p}}^{\text{3}}}$. So, arsenic has one three electron in p-orbital so, it accept three electrons from three fluorine atoms and ${\text{As}}{{\text{F}}_{\text{3}}}$ molecule. Now ${\text{As}}{{\text{F}}_{\text{3}}}$molecule has one lone pair so, it can work as electron donor but it also has vacant d-orbital so, it also accept electrons so, it is not only electron donor.
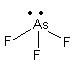
So, ${\text{N}}{{\text{H}}_{\text{3}}}$molecule is only an electron donor.
Therefore, option (A) ${\text{N}}{{\text{H}}_{\text{3}}}$is correct.
Note:In ${\text{B}}{{\text{F}}_{\text{3}}}$, after the formation of ${\text{B}}{{\text{F}}_{\text{3}}}$, the octet of boron does not get complete. Boron is able to accept two more electrons in ${\text{B}}{{\text{F}}_{\text{3}}}$. So, ${\text{B}}{{\text{F}}_{\text{3}}}$molecule is only an electron acceptor. The ${\text{P}}{{\text{F}}_{\text{3}}}$ and ${\text{As}}{{\text{F}}_{\text{3}}}$, have both have lone pair and vacant d-orbital so, both are electron donor as well as electron acceptor. The electrons donor substituents increase the electron donating power of a molecule whereas the presence of electron accepting substituents decrease the electron donating power of a molecule.
Complete answer:
A molecule that donates electrons is known as electron donor and the molecule which accepts electrons is known as electron acceptor.

${\text{N}}{{\text{H}}_{\text{3}}}$ known as ammonia has one lone pair on a nitrogen atom.
${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{3}}}$
The electronic configuration of nitrogen is . So, nitrogen has three electrons in p-orbital, so it accepts three electrons from three hydrogens and forms an ammonia molecule. Now there is no vacant p-orbital present in nitrogen so, ammonia molecule is only an electron donor because it can donate its lone pair but cannot accept electrons.

${\text{B}}{{\text{F}}_{\text{3}}}$, The electronic configuration of boron is ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^1}$. So, boron has only one electron in p-orbital so, it accept three electrons from three fluorine atoms and form ${\text{B}}{{\text{F}}_{\text{3}}}$molecule. ${\text{B}}{{\text{F}}_{\text{3}}}$molecule does not has any lone pair or negative charge, so it cannot work as an electron donor.

\[{\text{P}}{{\text{F}}_{\text{3}}}\], The electronic configuration of phosphorus is ${\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{3}}}{\text{3d}}$. So, phosphorus has one three electrons in p-orbital so, it accepts three electrons from three fluorine atoms and \[{\text{P}}{{\text{F}}_{\text{3}}}\] molecule. Now \[{\text{P}}{{\text{F}}_{\text{3}}}\]molecule has one lone pair so, it can work as electron donor but it also has vacant d-orbital so, it also accept electrons so, it is not only electron donor.

${\text{As}}{{\text{F}}_{\text{3}}}$, The electronic configuration of arsenic is ${\text{[Ar]3}}{{\text{d}}^{10}}{\text{4}}{{\text{s}}^2}{\text{4}}{{\text{p}}^{\text{3}}}$. So, arsenic has one three electron in p-orbital so, it accept three electrons from three fluorine atoms and ${\text{As}}{{\text{F}}_{\text{3}}}$ molecule. Now ${\text{As}}{{\text{F}}_{\text{3}}}$molecule has one lone pair so, it can work as electron donor but it also has vacant d-orbital so, it also accept electrons so, it is not only electron donor.

So, ${\text{N}}{{\text{H}}_{\text{3}}}$molecule is only an electron donor.
Therefore, option (A) ${\text{N}}{{\text{H}}_{\text{3}}}$is correct.
Note:In ${\text{B}}{{\text{F}}_{\text{3}}}$, after the formation of ${\text{B}}{{\text{F}}_{\text{3}}}$, the octet of boron does not get complete. Boron is able to accept two more electrons in ${\text{B}}{{\text{F}}_{\text{3}}}$. So, ${\text{B}}{{\text{F}}_{\text{3}}}$molecule is only an electron acceptor. The ${\text{P}}{{\text{F}}_{\text{3}}}$ and ${\text{As}}{{\text{F}}_{\text{3}}}$, have both have lone pair and vacant d-orbital so, both are electron donor as well as electron acceptor. The electrons donor substituents increase the electron donating power of a molecule whereas the presence of electron accepting substituents decrease the electron donating power of a molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




