
Which molecule is depicted by the given ball and stick models?
$
A.{\text{ (i)BeC}}{{\text{l}}_2}{\text{,(ii)C}}{{\text{H}}_4} \\
B.{\text{ (i)B}}{{\text{F}}_3}{\text{,(ii) PC}}{{\text{l}}_5} \\
{\text{C}}{\text{. (i)B}}{{\text{F}}_4}{\text{,(ii)C}}{{\text{H}}_4} \\
{\text{D}}{\text{. (i)BeC}}{{\text{l}}_2}{\text{,(ii)PC}}{{\text{l}}_5} \\
$

Answer
492.9k+ views
Hint :In chemistry the ball and stick model is a molecular model of chemical substance which is to display the three dimensional orientation of the structure and the type of bond present between the attached atom.
Complete Step By Step Answer:
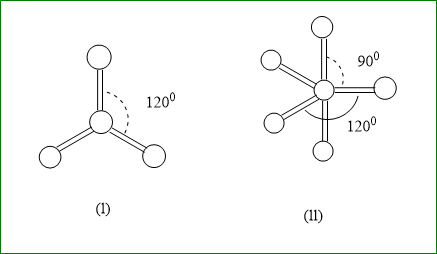
First, Let us understand a few concepts of what these balls and sticks are. The circular balls depicted in the diagram are the atoms and these sticks are the bond present between them. In this specific question, two lines represent only one bond because the diagrams are given in two-dimension.
Each central circle represents the central atom, which due to the larger number of valence shell electrons, have the ability to donate more electrons to side atoms which are lying on the sides.
In figure $ (i) $ , the total atoms are four amongst which three are side atoms and one is central atom.
We will consider only those substances, which have a total of four atoms in the question given.
From options given, $ BeC{l_2} $ has only two side atoms hence does not count for this figure. We can eliminate this option.
$ B{F_4},C{H_4},{\text{ and }}PC{l_5} $ , have more than four atoms in figure $ (i) $ .
So the only appropriate molecule is $ B{F_3} $ .
We will look for a figure $ (ii) $ , here a total of six atoms are there. One is the central atom and five side atoms. The only option with six atoms is $ PC{l_5} $ .
Hence the final answer will be option number $ (B.) $
Note :
This elimination method works only when the options are not given, sometimes they can also ask about $ B{F_3} $ and carbonate ions. This time one has to specifically write down the number of electrons and strictly adhere to it.
Complete Step By Step Answer:
First, Let us understand a few concepts of what these balls and sticks are. The circular balls depicted in the diagram are the atoms and these sticks are the bond present between them. In this specific question, two lines represent only one bond because the diagrams are given in two-dimension.
Each central circle represents the central atom, which due to the larger number of valence shell electrons, have the ability to donate more electrons to side atoms which are lying on the sides.
In figure $ (i) $ , the total atoms are four amongst which three are side atoms and one is central atom.
We will consider only those substances, which have a total of four atoms in the question given.
From options given, $ BeC{l_2} $ has only two side atoms hence does not count for this figure. We can eliminate this option.
$ B{F_4},C{H_4},{\text{ and }}PC{l_5} $ , have more than four atoms in figure $ (i) $ .
So the only appropriate molecule is $ B{F_3} $ .
We will look for a figure $ (ii) $ , here a total of six atoms are there. One is the central atom and five side atoms. The only option with six atoms is $ PC{l_5} $ .
Hence the final answer will be option number $ (B.) $
Note :
This elimination method works only when the options are not given, sometimes they can also ask about $ B{F_3} $ and carbonate ions. This time one has to specifically write down the number of electrons and strictly adhere to it.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

What is the difference between biodegradable and nonbiodegradable class 11 biology CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE




