
Which material is used for vulcanization of rubber?
A.Iron
B.Sulphur
C.Chlorine
D.Calcium carbonate
Answer
579.9k+ views
Hint: Basically, vulcanization refers to a range of processes for hardening rubbers. It generally refers to the treatment of natural rubber with Sulphur. To solve this question, we need to know about the different properties and processes involved in vulcanization of rubber.
Complete step by step answer:
Rubber is an elastic substance which can be obtained naturally or artificially. There are two primary types of rubber namely natural rubber and synthetic rubber. Natural rubber is made up of solid particles suspended in a milky white liquid called latex. It is generally prepared by rubber tapping, mastication and calendaring. Now performing all these steps will not yield rubber that is strong or hard enough to be used in items like car tires and machinery. So, to enhance these properties, Sulphur is added to the rubber and it is heated at a temperature ranging from 373k to 415k. This process is known as vulcanization.
Moreover, the Sulphur acts as a crosslinking agent and after vulcanization, rubber gets cross-lined and becomes hard. The main polymers subjected to sulfur vulcanization are polyisoprene and styrene butadiene rubber which are used for most of the street-vehicles tires. The process is as shown:
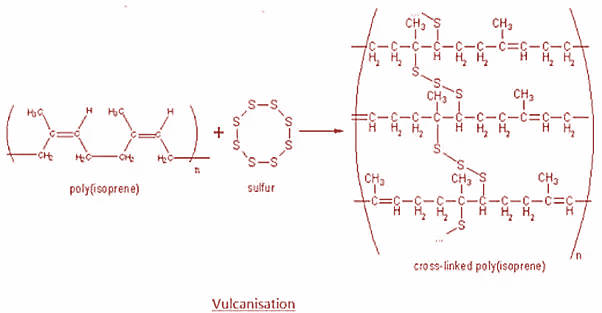
Hence, option B is correct.
Note: Synthetic rubbers are basically produced from petroleum and natural gas. Moreover, rubbers has various applications. In the clothing industry, they are used as wetsuits and expandable clothes such as gym and cycling shorts. They are also used for flooring purposes, in airbags, tires etc.
Complete step by step answer:
Rubber is an elastic substance which can be obtained naturally or artificially. There are two primary types of rubber namely natural rubber and synthetic rubber. Natural rubber is made up of solid particles suspended in a milky white liquid called latex. It is generally prepared by rubber tapping, mastication and calendaring. Now performing all these steps will not yield rubber that is strong or hard enough to be used in items like car tires and machinery. So, to enhance these properties, Sulphur is added to the rubber and it is heated at a temperature ranging from 373k to 415k. This process is known as vulcanization.
Moreover, the Sulphur acts as a crosslinking agent and after vulcanization, rubber gets cross-lined and becomes hard. The main polymers subjected to sulfur vulcanization are polyisoprene and styrene butadiene rubber which are used for most of the street-vehicles tires. The process is as shown:

Hence, option B is correct.
Note: Synthetic rubbers are basically produced from petroleum and natural gas. Moreover, rubbers has various applications. In the clothing industry, they are used as wetsuits and expandable clothes such as gym and cycling shorts. They are also used for flooring purposes, in airbags, tires etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




