
Which is the main product of the following reaction?
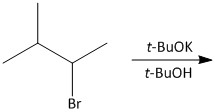
A.
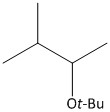
B.
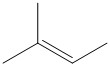
C.
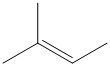
D.





Answer
578.4k+ views
Hint: The given substrate is an alkyl halide and the halide is attached to a secondary carbon atom. The base used is potassium t-butoxide which is a very strong base for proton abstraction.
Complete step by step answer:
When secondary alkyl halides attached to two different adjacent \[C - H\] groups are treated with a strong base two products are possible. One is the Saytzeff product and the other is the Hofmann product.
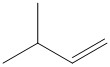
The reaction conditions and the bulk of the reagents and substrate are responsible for the formation of the most favored product. In general the product formed by following Saytzeff’s rule is thermodynamically more stable as the olefin formed by elimination is highly substituted. For the given substrate the following olefin is the Saytzeff product.
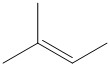
The double bond is attached to three methyl substituents and has more number of hyperconjugable hydrogen atoms.
However in case of steric interaction between the substrate and the bulk of the reagent, Hofmann product becomes favorable.
For the given substrate, the base used for elimination is potassium t-butoxide which is a bulky base. It will experience steric interaction with the methyl group present at the \[C3\] position. So the base will mostly abstract the proton present at the \[C1\] position and lead to the formation of the Hofmann product. The product formed by abstracting the proton \[C3\] product will be minor. The mechanism of elimination can be shown as:
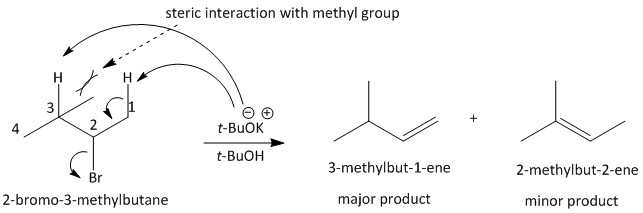
Thus the main product of the reaction is \[3\]-methylbut-\[1\]-ene, i.e. option D is the correct answer.
Note:
The minor product can be favored by changing the base used for the reaction. If a smaller base like sodium methoxide is used it will abstract the \[C3\] proton more than the \[C1\] proton and then the Saytzeff product will be major and Hofmann product will be minor.
Complete step by step answer:
When secondary alkyl halides attached to two different adjacent \[C - H\] groups are treated with a strong base two products are possible. One is the Saytzeff product and the other is the Hofmann product.
The reaction conditions and the bulk of the reagents and substrate are responsible for the formation of the most favored product. In general the product formed by following Saytzeff’s rule is thermodynamically more stable as the olefin formed by elimination is highly substituted. For the given substrate the following olefin is the Saytzeff product.

The double bond is attached to three methyl substituents and has more number of hyperconjugable hydrogen atoms.
However in case of steric interaction between the substrate and the bulk of the reagent, Hofmann product becomes favorable.
For the given substrate, the base used for elimination is potassium t-butoxide which is a bulky base. It will experience steric interaction with the methyl group present at the \[C3\] position. So the base will mostly abstract the proton present at the \[C1\] position and lead to the formation of the Hofmann product. The product formed by abstracting the proton \[C3\] product will be minor. The mechanism of elimination can be shown as:

Thus the main product of the reaction is \[3\]-methylbut-\[1\]-ene, i.e. option D is the correct answer.
Note:
The minor product can be favored by changing the base used for the reaction. If a smaller base like sodium methoxide is used it will abstract the \[C3\] proton more than the \[C1\] proton and then the Saytzeff product will be major and Hofmann product will be minor.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




