
Which is not true about $Si{{O}_{2}}$?
(A) It is a network solid
(B) It is attacked by molten NaOH
(C) It is attacked by HF
(D) It is the basic structural unit of silicates
Answer
529.5k+ views
Hint: To solve this question, we first need to know what is $Si{{O}_{2}}$. Silicon dioxide ($Si{{O}_{2}}$) or silica is a white amorphous chemical compound and an oxide of silicon. It is found in living organisms and exists in nature as quartz.
Complete answer:
Let us scrutinize the given statements
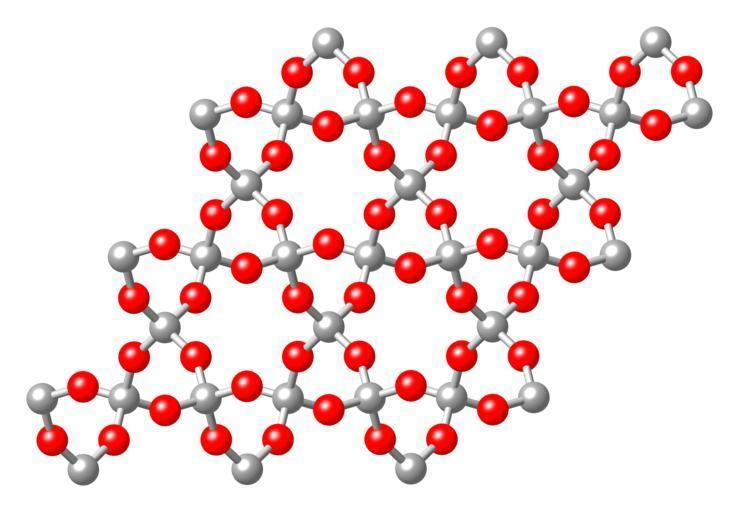
(A) The statement that silicon dioxide ($Si{{O}_{2}}$) is a network solid is true.
When the atoms are bonded by covalent bonds in a chemical compound in a continuous network that extends throughout the material is known as a network solid or an atomic crystalline solid.
Silicon dioxide ($Si{{O}_{2}}$) has a three-dimensional continuous network of $Si{{O}_{2}}$ units and hence it is a network solid.
(B) The statement that silicon dioxide ($Si{{O}_{2}}$) is attacked by molten NaOH is true.
When hot and molten (i.e., concentrated) sodium hydroxide (NaOH) reacts with silicon dioxide ($Si{{O}_{2}}$) at a temperature of 900-1000${}^\circ C$, sodium silicate ($N{{a}_{2}}Si{{O}_{3}}$) is formed along with water molecule. The reaction proceeds as follows:
\[Si{{O}_{2}}+2NaOH\xrightarrow{900-1000{}^\circ C}N{{a}_{2}}Si{{O}_{3}}+{{H}_{2}}O\]
(C) The statement that silicon dioxide ($Si{{O}_{2}}$) is attacked by HF is true.
When hydrogen fluoride (HF) attacks silicon dioxide ($Si{{O}_{2}}$), hexafluorosilicic acid (${{H}_{2}}Si{{F}_{6}}$ ) is produced along with the formation of water molecules. The reaction proceeds as follows:
\[Si{{O}_{2}}+6HF\to {{H}_{2}}Si{{F}_{6}}+2{{H}_{2}}O\]
(D) The statement that silicon dioxide ($Si{{O}_{2}}$) is the basic structural unit of silicates is false.
The basic structural units of silicates are $SiO_{4}^{4-}$.
So, the answer to the question is option (D).
Note:
It should be noted that
- Silicon can be produced by reducing silicon dioxide ($Si{{O}_{2}}$) with carbon.
- Halogen gases except fluorine do not essentially react with silicon dioxide ($Si{{O}_{2}}$).
- Silicon dioxide ($Si{{O}_{2}}$) does not react with most acids.
- Silicon dioxide ($Si{{O}_{2}}$) is insoluble in water but dissolves in hydrofluoric acid (HF).
Complete answer:
Let us scrutinize the given statements
(A) The statement that silicon dioxide ($Si{{O}_{2}}$) is a network solid is true.
When the atoms are bonded by covalent bonds in a chemical compound in a continuous network that extends throughout the material is known as a network solid or an atomic crystalline solid.
Silicon dioxide ($Si{{O}_{2}}$) has a three-dimensional continuous network of $Si{{O}_{2}}$ units and hence it is a network solid.

(B) The statement that silicon dioxide ($Si{{O}_{2}}$) is attacked by molten NaOH is true.
When hot and molten (i.e., concentrated) sodium hydroxide (NaOH) reacts with silicon dioxide ($Si{{O}_{2}}$) at a temperature of 900-1000${}^\circ C$, sodium silicate ($N{{a}_{2}}Si{{O}_{3}}$) is formed along with water molecule. The reaction proceeds as follows:
\[Si{{O}_{2}}+2NaOH\xrightarrow{900-1000{}^\circ C}N{{a}_{2}}Si{{O}_{3}}+{{H}_{2}}O\]
(C) The statement that silicon dioxide ($Si{{O}_{2}}$) is attacked by HF is true.
When hydrogen fluoride (HF) attacks silicon dioxide ($Si{{O}_{2}}$), hexafluorosilicic acid (${{H}_{2}}Si{{F}_{6}}$ ) is produced along with the formation of water molecules. The reaction proceeds as follows:
\[Si{{O}_{2}}+6HF\to {{H}_{2}}Si{{F}_{6}}+2{{H}_{2}}O\]
(D) The statement that silicon dioxide ($Si{{O}_{2}}$) is the basic structural unit of silicates is false.
The basic structural units of silicates are $SiO_{4}^{4-}$.
So, the answer to the question is option (D).
Note:
It should be noted that
- Silicon can be produced by reducing silicon dioxide ($Si{{O}_{2}}$) with carbon.
- Halogen gases except fluorine do not essentially react with silicon dioxide ($Si{{O}_{2}}$).
- Silicon dioxide ($Si{{O}_{2}}$) does not react with most acids.
- Silicon dioxide ($Si{{O}_{2}}$) is insoluble in water but dissolves in hydrofluoric acid (HF).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




