
Which is not the resonance structure of aniline?
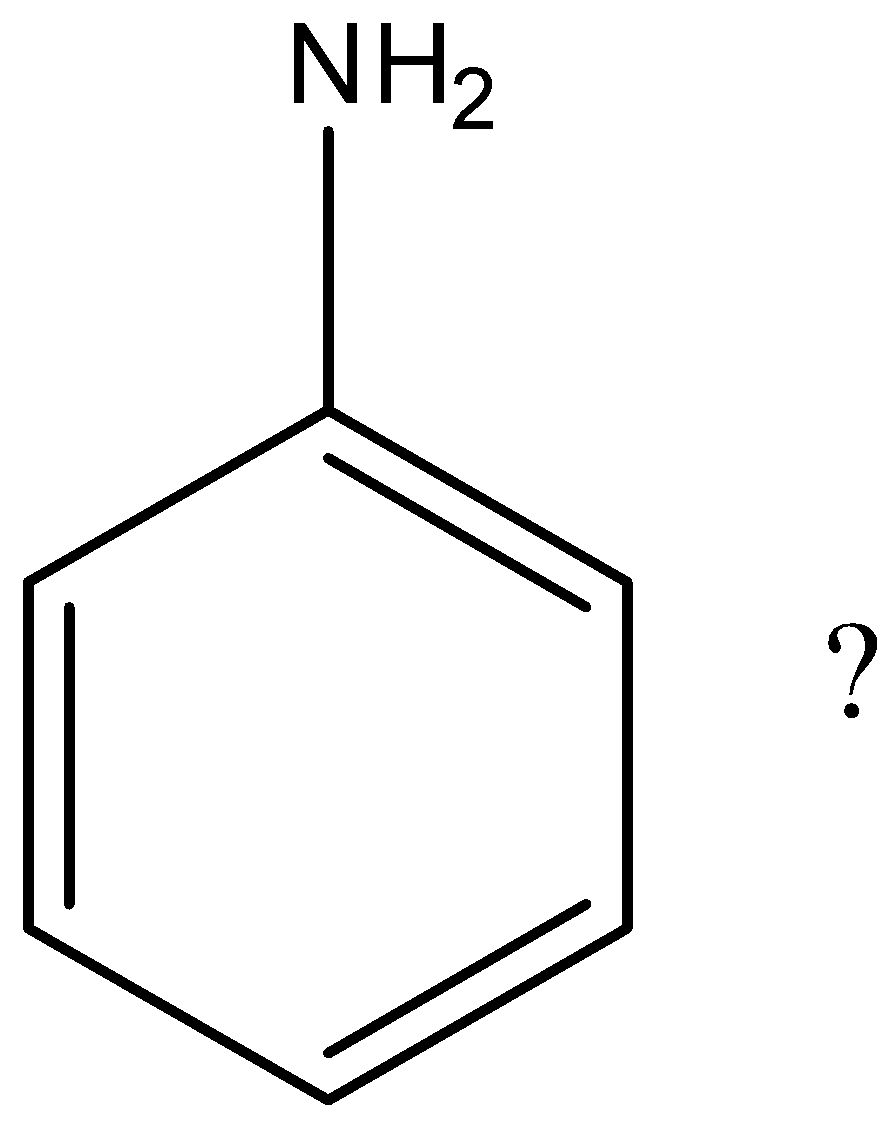
A.
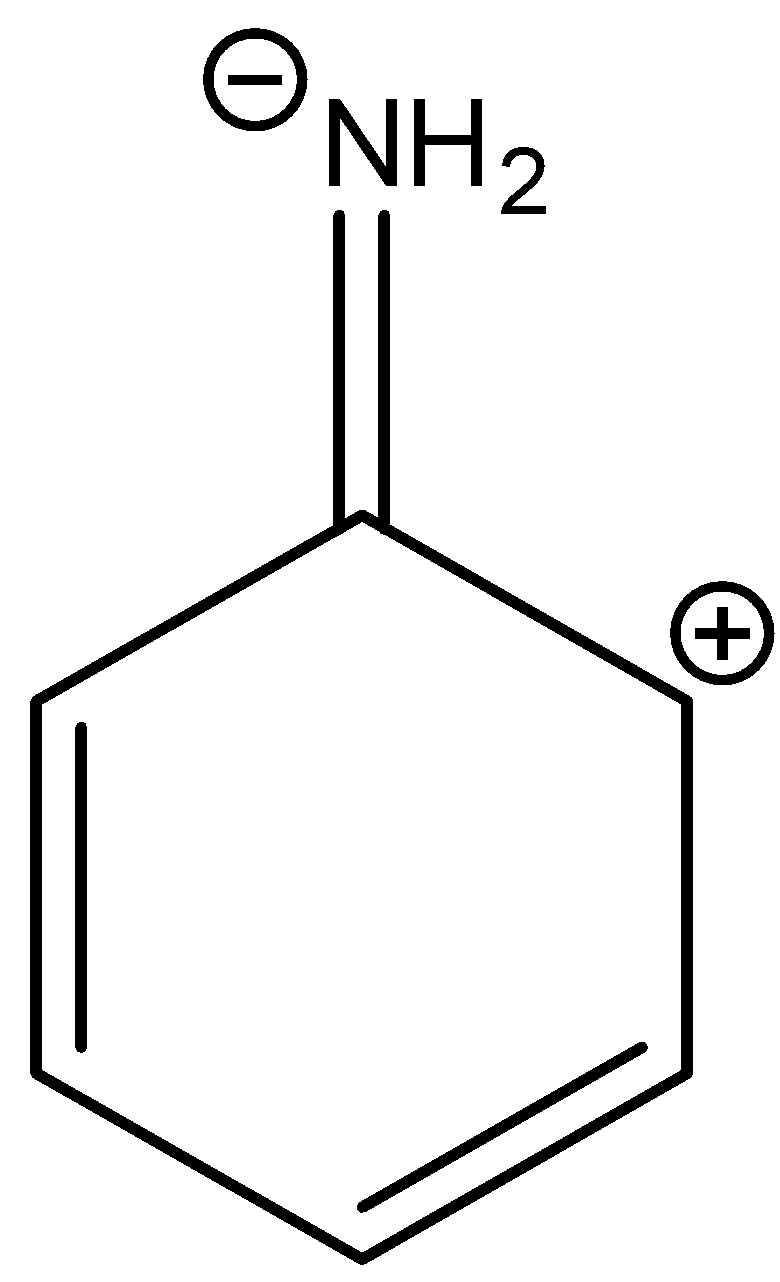
B.
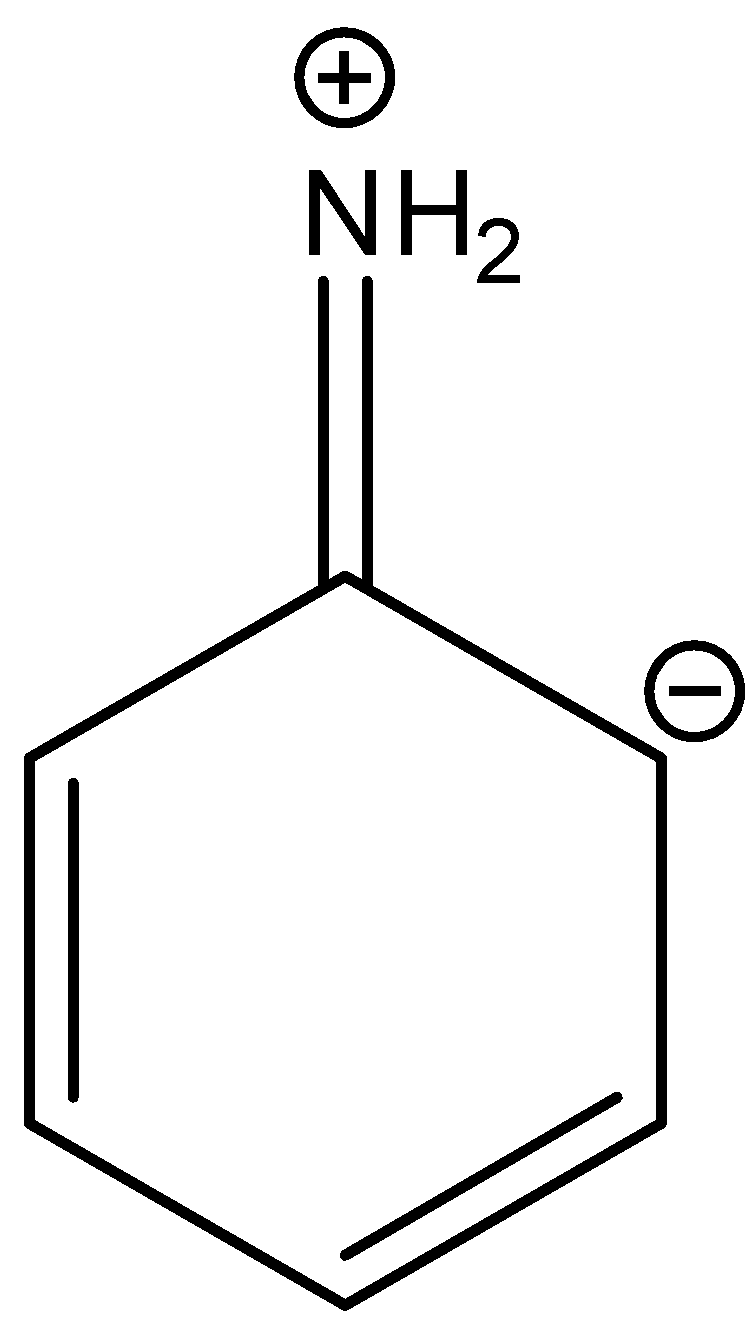
C.
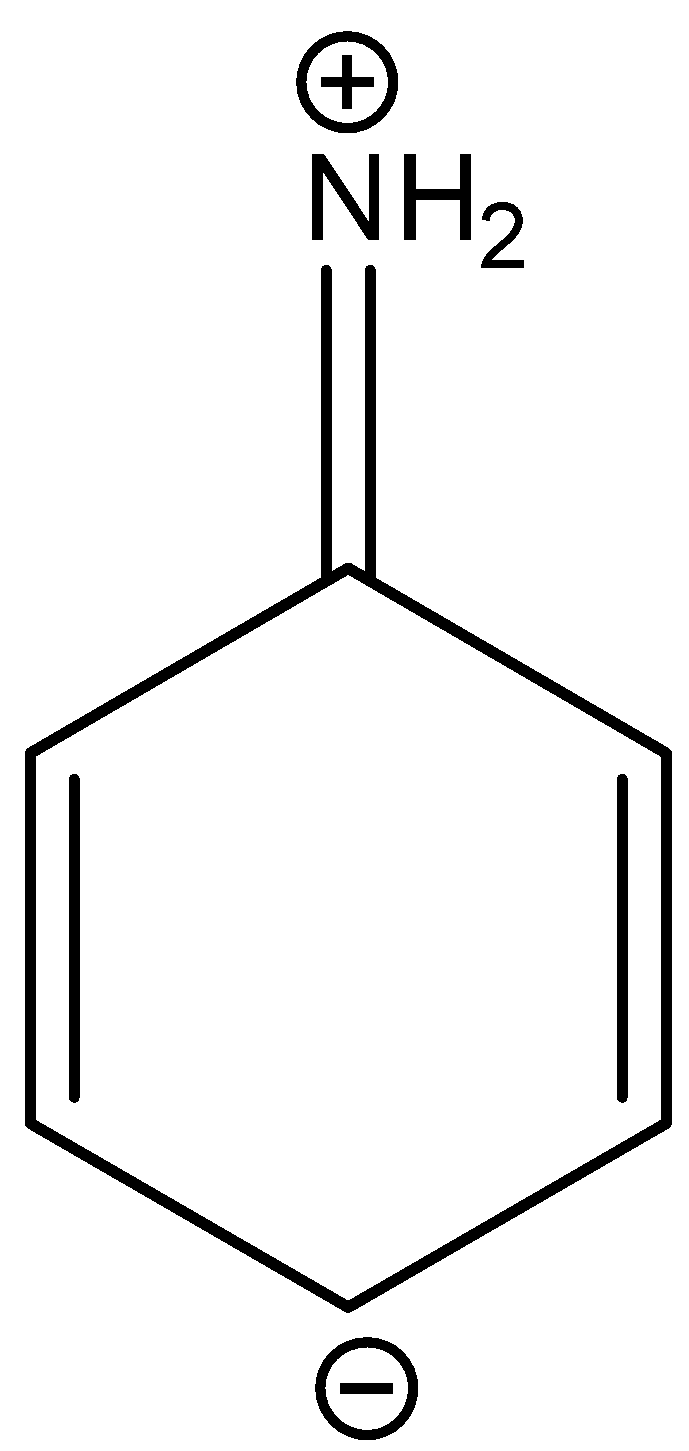
D.





Answer
588k+ views
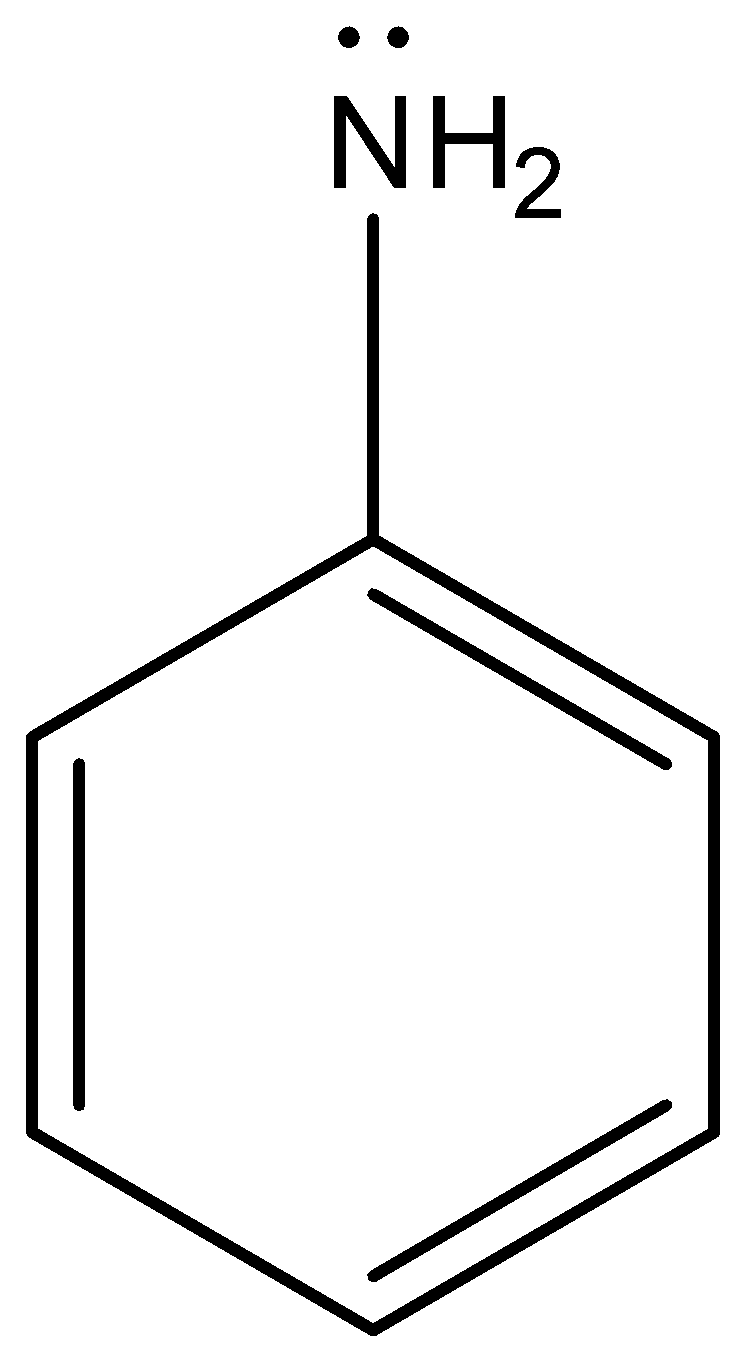
Hint: Aniline is an organic compound that has an aromatic ring in its structure. Aniline consists of a phenyl ring attached to an amine group. The structure of aniline is as follows. Due to the presence of lone pairs of electrons on nitrogen in amine groups of aniline undergoes resonance with the aromatic ring.
Complete step by step answer:
Aniline shows resonance structures due to the presence of a lone pair of electrons present in the anime group which is attached to a benzene ring.
The resonance structure of the aniline is as follows.
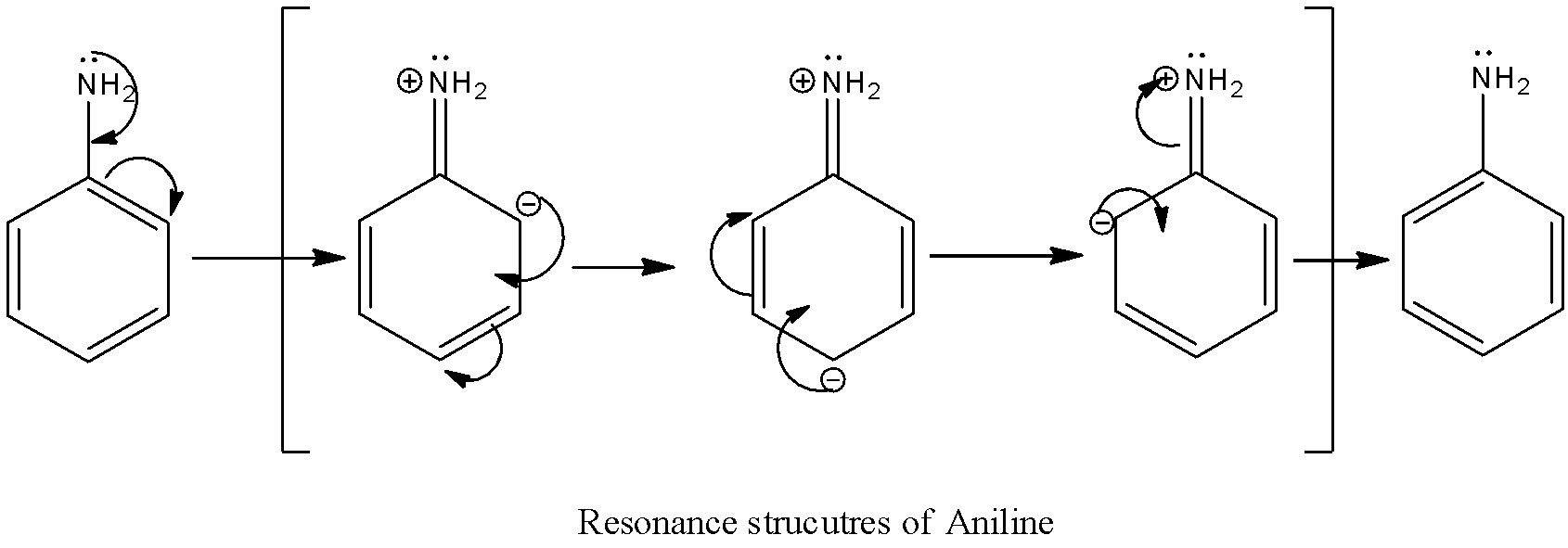
There are three resonance structures for aniline.
The resonance structures of aniline are due to the involvement of lone pairs of electrons on nitrogen.
In three resonance structures of aniline, the Nitrogen atom in amine group has a positive charge and one of the Carbon atoms in the ring (ortho or para to amine group in aniline) contains a negative charge.
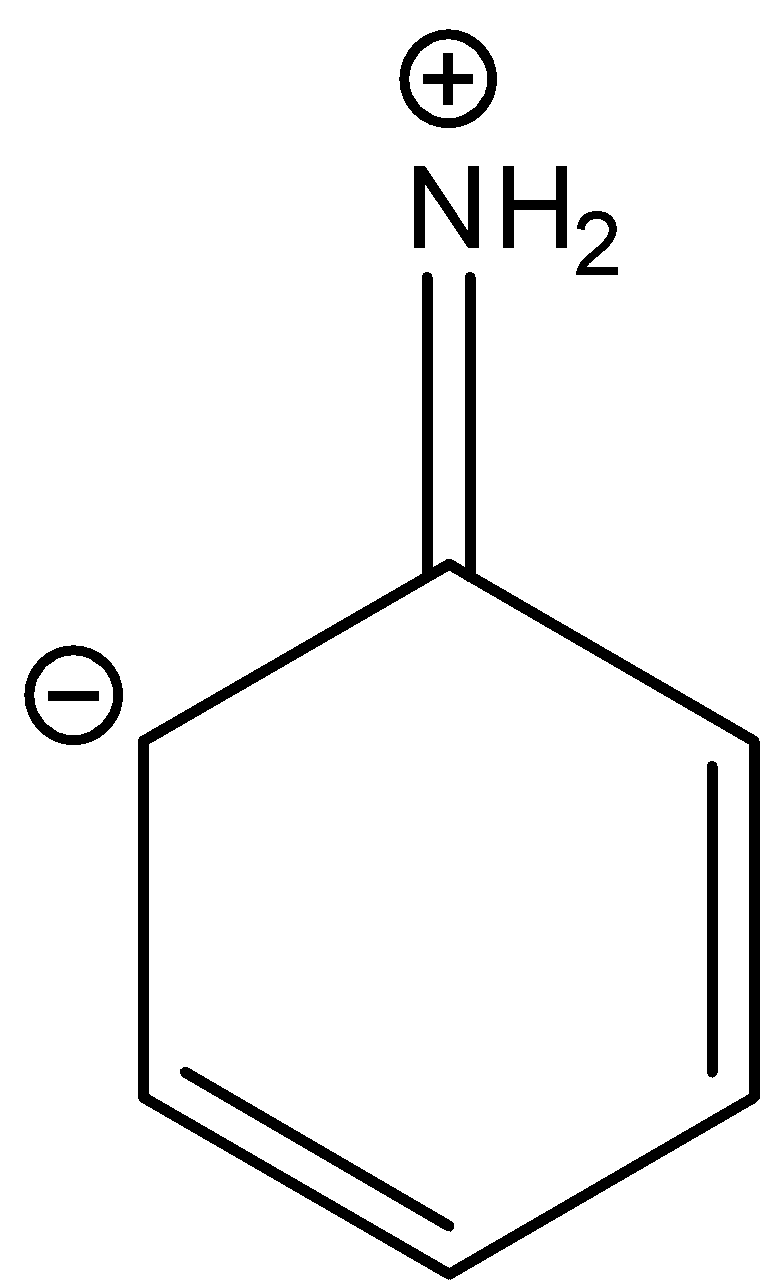
There is no resonance structure containing a negative charge on nitrogen in amine groups. But in option A there is a negative charge on the nitrogen atom in the amine group.
So, the correct answer is “Option A”.
Note: In the resonance structures of aniline the negative charge is present at ortho and para positions to the amine group. Because of the presence of negative charge in aniline, electrophiles are going to react with aniline easily at ortho and para positions. So, amine in aniline directs electrophiles ortho or para positions. Therefore the amine group is called ortho or para directing group.

Complete step by step answer:
Aniline shows resonance structures due to the presence of a lone pair of electrons present in the anime group which is attached to a benzene ring.
The resonance structure of the aniline is as follows.

There are three resonance structures for aniline.
The resonance structures of aniline are due to the involvement of lone pairs of electrons on nitrogen.
In three resonance structures of aniline, the Nitrogen atom in amine group has a positive charge and one of the Carbon atoms in the ring (ortho or para to amine group in aniline) contains a negative charge.
There is no resonance structure containing a negative charge on nitrogen in amine groups. But in option A there is a negative charge on the nitrogen atom in the amine group.
So, the correct answer is “Option A”.
Note: In the resonance structures of aniline the negative charge is present at ortho and para positions to the amine group. Because of the presence of negative charge in aniline, electrophiles are going to react with aniline easily at ortho and para positions. So, amine in aniline directs electrophiles ortho or para positions. Therefore the amine group is called ortho or para directing group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




