
Which is having non zero dipole moment?
A. \[{{H}_{2}}O\]
B. Pytene
C. Chloroform
D. Ethene
Answer
560.4k+ views
Hint: The bond dipole moment tends to use the idea of an electric dipole moment to determine the polarity of the chemical bond which is present in the molecule. The dipole moment occurs when there is the charge separation present. The bond dipole moment is the vector quantity whose direction is defined as the parallel to the bond axis.
Complete answer:
So in option a that is in water molecules we see that the localisation of electrons is around the oxygen atom because it is more electronegative in nature in comparison to hydrogen atom. But as we know that there is a presence of the lone pairs of electrons in the oxygen atom which causes the water molecule to get a bent shape according to the VSEPR theory. Due to which the individual bond dipole moments do not get canceled with each other. The illustration which will help to describe the dipole moment in water is given below:
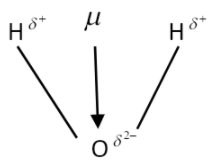
The bond angle of water is\[{{104.5}^{o}}\] and the individual bond moment of the oxygen hydrogen bond is 1.5D and so the net dipole moment is 1.84D in water molecules. it is triangular in structure and the dipole bonds are equal in magnitude but opposite in direction. So it has a non zero dipole moment. While the other options that are pythene, chloroform and ethanol become zero as the dipole bonds are equal in magnitude as well as not opposing each other in direction.
Hence the correct option is option A.
Note: Dipole moment is used for determining the polarity of the bond. The dipole moment usually depends on two things and they are the arrangement of the molecules and the individual dipole moment of each bond. The molecule with higher dipole moment has higher polarity of bonds in its molecule.
Complete answer:
So in option a that is in water molecules we see that the localisation of electrons is around the oxygen atom because it is more electronegative in nature in comparison to hydrogen atom. But as we know that there is a presence of the lone pairs of electrons in the oxygen atom which causes the water molecule to get a bent shape according to the VSEPR theory. Due to which the individual bond dipole moments do not get canceled with each other. The illustration which will help to describe the dipole moment in water is given below:

The bond angle of water is\[{{104.5}^{o}}\] and the individual bond moment of the oxygen hydrogen bond is 1.5D and so the net dipole moment is 1.84D in water molecules. it is triangular in structure and the dipole bonds are equal in magnitude but opposite in direction. So it has a non zero dipole moment. While the other options that are pythene, chloroform and ethanol become zero as the dipole bonds are equal in magnitude as well as not opposing each other in direction.
Hence the correct option is option A.
Note: Dipole moment is used for determining the polarity of the bond. The dipole moment usually depends on two things and they are the arrangement of the molecules and the individual dipole moment of each bond. The molecule with higher dipole moment has higher polarity of bonds in its molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




