
Which functional group is introduced in phenol when it reacts with chloroform and dilute sodium hydroxide?
A. $ - CHC{l_2}$
B. $ - CHO$
C. $ - C{H_2}Cl$
D. $ - COOH$
Answer
569.7k+ views
Hint:When phenol that is ${C_6}{H_6}O$ reacts with chloroform that is and sodium hydroxide that is $NaOH$, it is called Reimer–Tiemann reaction. This reaction introduces the aldehyde group in phenol $CHC{l_3}$ at ortho position. At ortho position, intramolecular $H$ bonding occurs.
Complete answer:
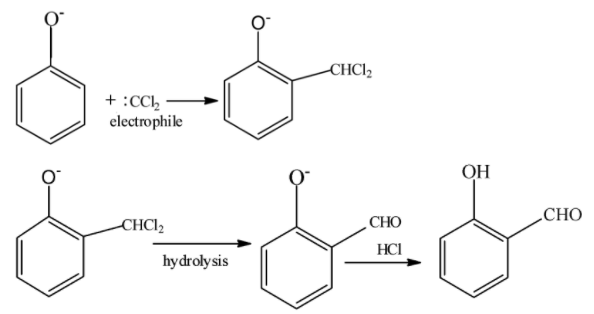
The Reimer–Tiemann reaction is a very famous reaction which is used to convert phenol ${C_6}{H_6}O$ into salicylaldehyde ${C_7}{H_6}{O_2}$ . It was discovered by Karl Reimer and Ferdinand Tiemann. This reaction has two steps .In first step phenol reacts with chloroform $CHC{l_3}$ and $NaOH$ to produce an intermediate .In the second step this intermediate undergoes hydrolysis to give $2$-hydroxy benzaldehyde.
It is important we understand the mechanism of the reaction . In the first step of the mechanism $O{H^ - }$ of $NaOH$ reacts with $H$of $CHC{l_3}$ to form water and trichloromethyl anion. It is called deprotonation. Similarly deprotonation of phenol occurs.
$CHC{l_3} + O{H^ - } \to {H_2}O + CC{l_3}^-$
Since chlorine is a group $17$ element and highly electronegative, it is a very good leaving group .So therefore chlorine leaves trichloromethyl anion and negative charge on trichloromethyl anion is neutralized. The product formed in the second step reacts with deprotonated phenol to form an intermediate product. This further undergoes hydrolysis to give salicylaldehyde. The product formed in the second step reacts with deprotonated phenol to form an intermediate product. This further undergoes hydrolysis to give salicylaldehyde.
So the correct option is $B$
Note:
It is important to note that instead of $NaOH$ we can also use $KOH$. $NaOH$ or $KOH$ helps in deprotonation of chloroform and phenol. So it becomes necessary to use a strong base to begin the reaction.
Complete answer:
The Reimer–Tiemann reaction is a very famous reaction which is used to convert phenol ${C_6}{H_6}O$ into salicylaldehyde ${C_7}{H_6}{O_2}$ . It was discovered by Karl Reimer and Ferdinand Tiemann. This reaction has two steps .In first step phenol reacts with chloroform $CHC{l_3}$ and $NaOH$ to produce an intermediate .In the second step this intermediate undergoes hydrolysis to give $2$-hydroxy benzaldehyde.
It is important we understand the mechanism of the reaction . In the first step of the mechanism $O{H^ - }$ of $NaOH$ reacts with $H$of $CHC{l_3}$ to form water and trichloromethyl anion. It is called deprotonation. Similarly deprotonation of phenol occurs.
$CHC{l_3} + O{H^ - } \to {H_2}O + CC{l_3}^-$
Since chlorine is a group $17$ element and highly electronegative, it is a very good leaving group .So therefore chlorine leaves trichloromethyl anion and negative charge on trichloromethyl anion is neutralized. The product formed in the second step reacts with deprotonated phenol to form an intermediate product. This further undergoes hydrolysis to give salicylaldehyde. The product formed in the second step reacts with deprotonated phenol to form an intermediate product. This further undergoes hydrolysis to give salicylaldehyde.

So the correct option is $B$
Note:
It is important to note that instead of $NaOH$ we can also use $KOH$. $NaOH$ or $KOH$ helps in deprotonation of chloroform and phenol. So it becomes necessary to use a strong base to begin the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




