
Which compound react with alkaline aqueous iodine to give a pale-yellow precipitate of tri-iodomethane?
1.butanone
2.ethanal
3.propan-2-ol
A. 1, 2 and 3 are correct
B. 1 and 2 only are correct
C. 2 and 3 only are correct
D. 1 only is correct
Answer
579.6k+ views
Hint: Any compounds containing the $C{H_3}C = 0$ group or the $C{H_3}CH(OH)$ group will give a positive result with the iodoform test. When ${I_2}$ and NaOH is added to a compound containing one of these groups, then a pale-yellow precipitate of iodoform (triiodomethane) is formed.
Complete step by step answer:
When Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, it gives a positive result - a pale yellow precipitate of iodoform or triiodomethane is formed.
Tri-iodomethane (iodoform) reaction shows a positive result – a pale yellow precipitate of triiodomethane (iodoform) is given by an aldehyde or ketone containing the grouping:
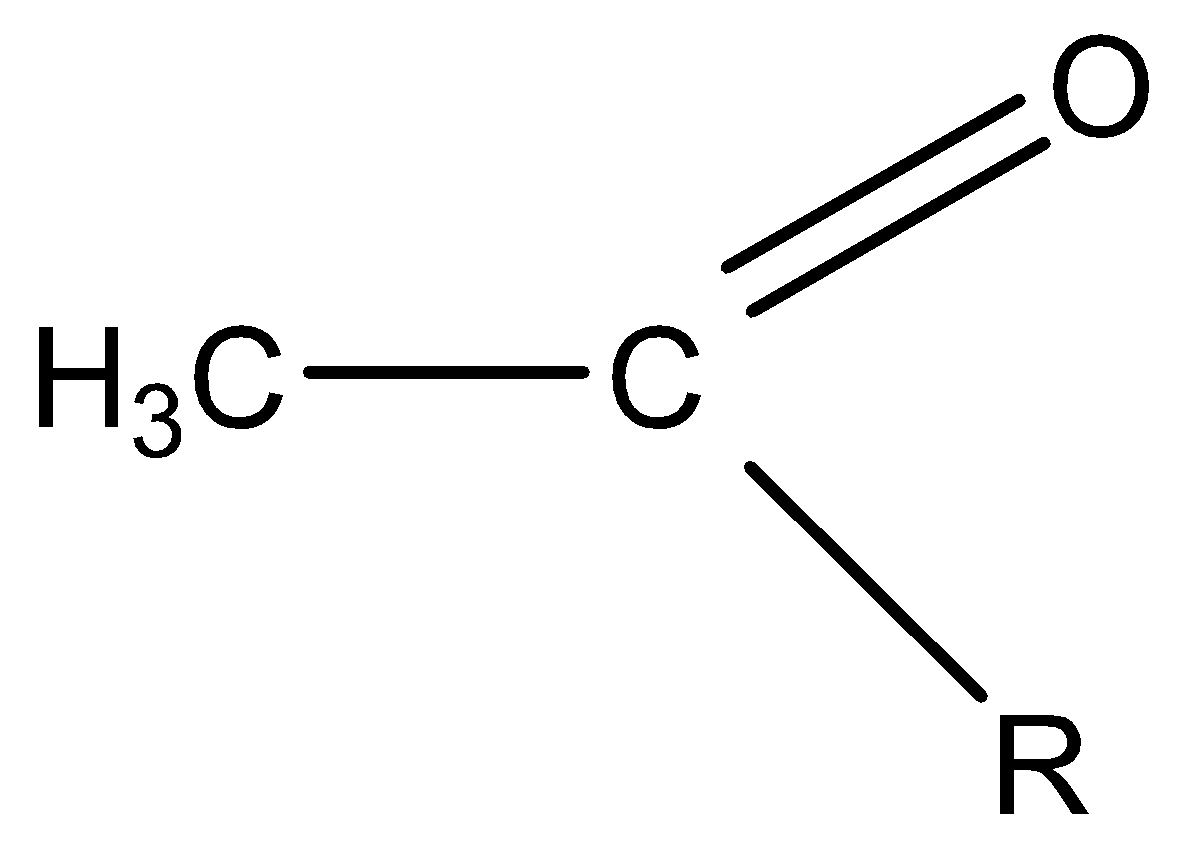
“R” can be a hydrogen atom or a hydrocarbon group (for example, an alkyl group).
If R is hydrogen, then we have the aldehyde ethanal, $C{H_3}CHO$ . Ethanal is the only aldehyde to give a triiodomethane reaction.
If R is a hydrocarbon group, then we have a ketone. Many ketones give this reaction, but all have a methyl group on one side of the carbon-oxygen bond. These are known as methyl ketones. And butanone is a methyl ethyl ketone $(C{H_3}(C = O)C{H_2}C{H_3})$ .
This test can also be used to identify alcohols $(C{H_3}C{H_2}OH)$ . If the alcohol is a tertiary alcohol then it does not give yellow precipitate as it cannot be oxidised. If the alcohol is a primary alcohol then it must be ethanol (as this is oxidised to ethanal, which is the only aldehyde that gives a positive result with the iodoform test). All secondary alcohols give a positive result, as they are oxidised to ketones. And propan-2-ol is a secondary alcohol.
So, all the compounds above i.e. butanone, Ethanal and propan-2-ol react with alkaline aqueous iodine to give a pale-yellow precipitate of tri-iodomethane.
Therefore, the correct answer is option (A).
Note: The iodoform test can be used to identify only ethanal if it is an aldehyde (this is the only aldehyde with the $C{H_3}CHO$ group). This occurs as three I atoms replace the H atoms of $C{H_3}C = OR$ , and the \[C - C\] bond breaks due to the electron withdrawing effect of the three I atoms (as I is more electronegative than C) forming $C{H_3}I$ and the salt anion of a carboxylic acid (depending on the R group of the original compound, which influences the length of the carbon chain of the anion \[RCOO - \] that is formed).
Complete step by step answer:
When Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, it gives a positive result - a pale yellow precipitate of iodoform or triiodomethane is formed.
Tri-iodomethane (iodoform) reaction shows a positive result – a pale yellow precipitate of triiodomethane (iodoform) is given by an aldehyde or ketone containing the grouping:

“R” can be a hydrogen atom or a hydrocarbon group (for example, an alkyl group).
If R is hydrogen, then we have the aldehyde ethanal, $C{H_3}CHO$ . Ethanal is the only aldehyde to give a triiodomethane reaction.
If R is a hydrocarbon group, then we have a ketone. Many ketones give this reaction, but all have a methyl group on one side of the carbon-oxygen bond. These are known as methyl ketones. And butanone is a methyl ethyl ketone $(C{H_3}(C = O)C{H_2}C{H_3})$ .
This test can also be used to identify alcohols $(C{H_3}C{H_2}OH)$ . If the alcohol is a tertiary alcohol then it does not give yellow precipitate as it cannot be oxidised. If the alcohol is a primary alcohol then it must be ethanol (as this is oxidised to ethanal, which is the only aldehyde that gives a positive result with the iodoform test). All secondary alcohols give a positive result, as they are oxidised to ketones. And propan-2-ol is a secondary alcohol.
So, all the compounds above i.e. butanone, Ethanal and propan-2-ol react with alkaline aqueous iodine to give a pale-yellow precipitate of tri-iodomethane.
Therefore, the correct answer is option (A).
Note: The iodoform test can be used to identify only ethanal if it is an aldehyde (this is the only aldehyde with the $C{H_3}CHO$ group). This occurs as three I atoms replace the H atoms of $C{H_3}C = OR$ , and the \[C - C\] bond breaks due to the electron withdrawing effect of the three I atoms (as I is more electronegative than C) forming $C{H_3}I$ and the salt anion of a carboxylic acid (depending on the R group of the original compound, which influences the length of the carbon chain of the anion \[RCOO - \] that is formed).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




