
Which compound is formed when a mixture of calcium acetate and calcium formate is heated?
A.
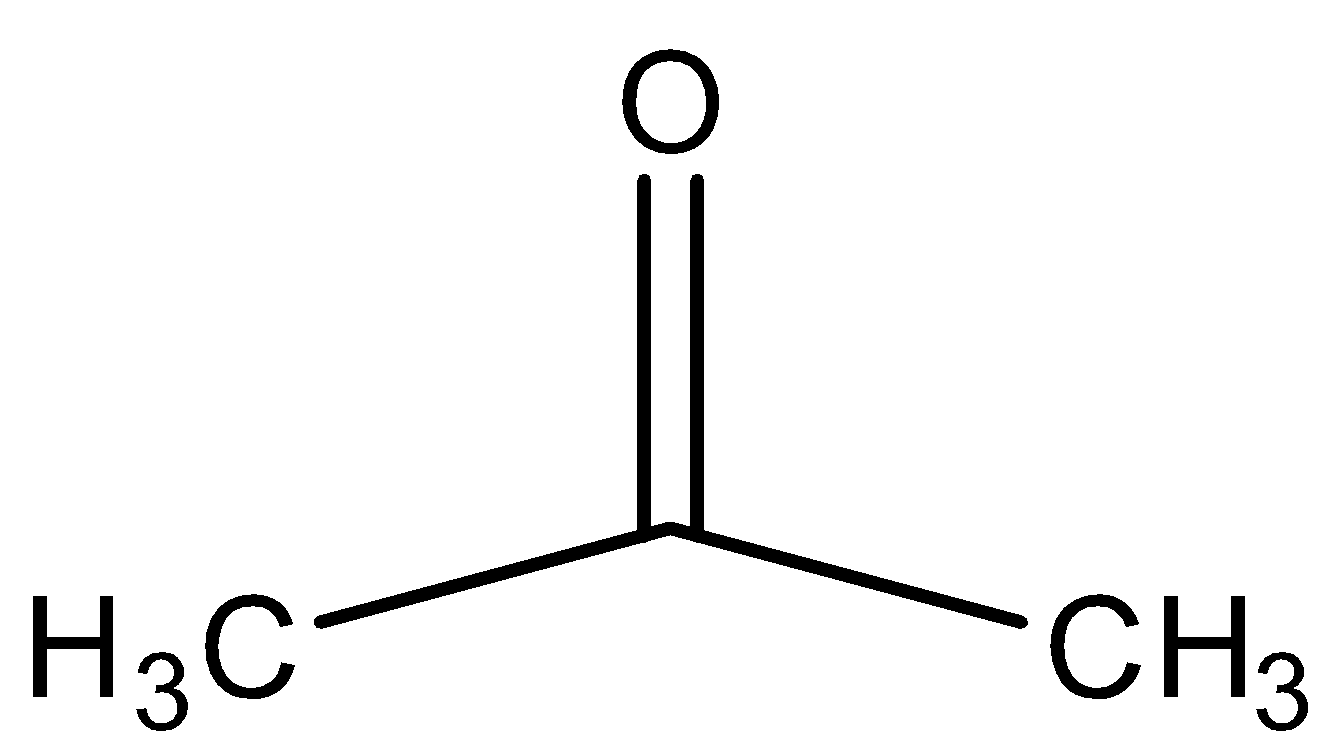
B.
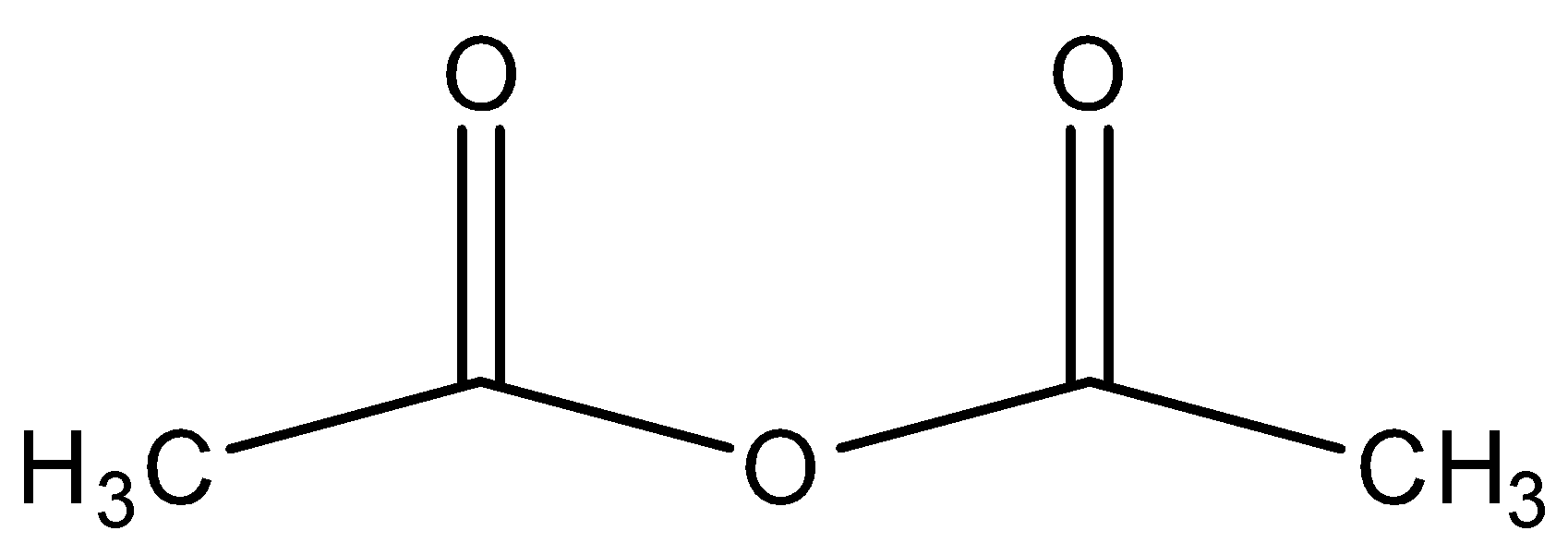
C.
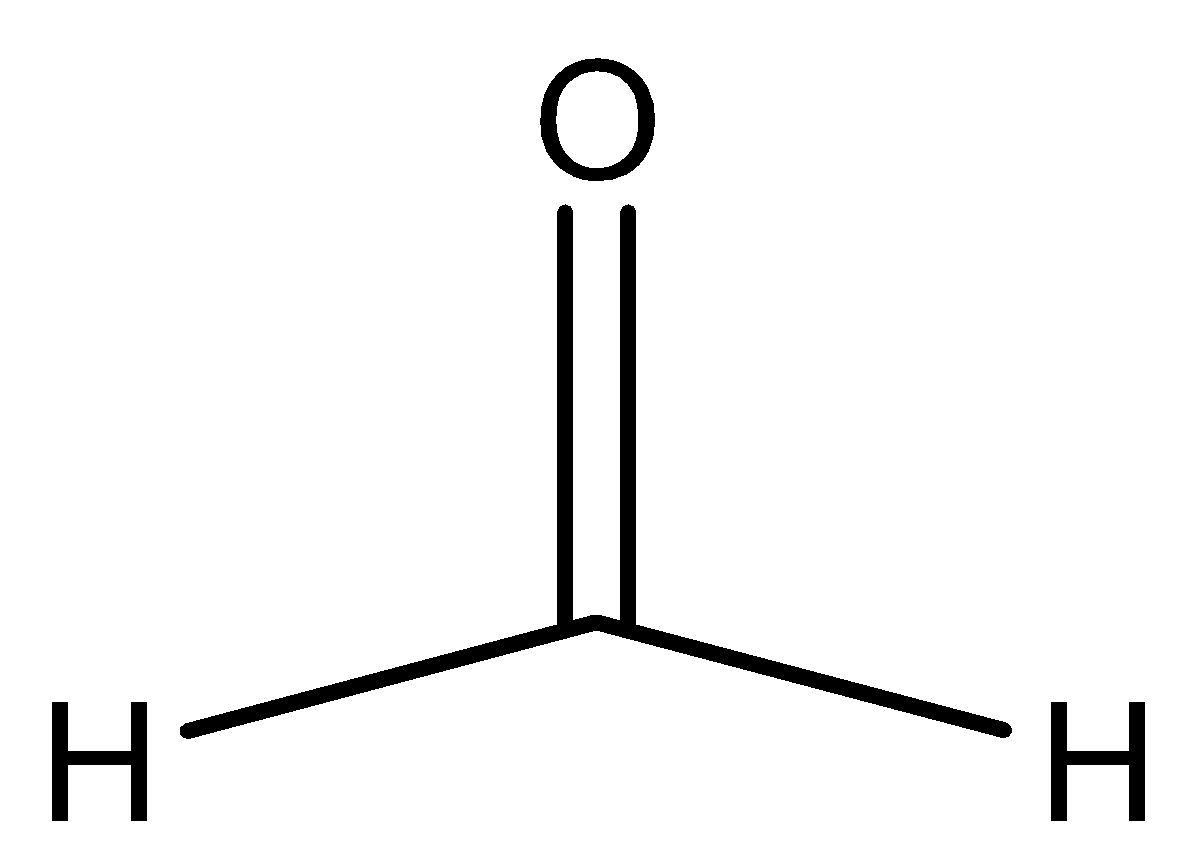
D.
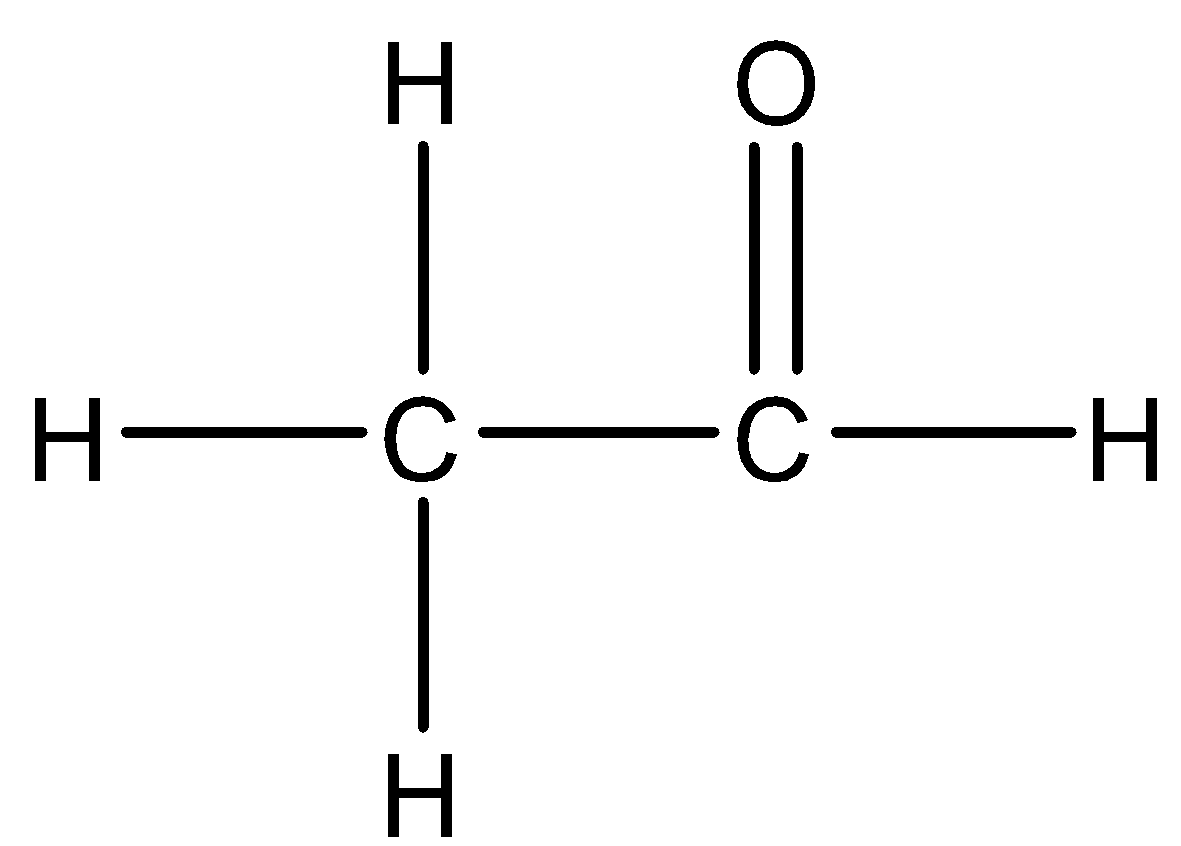




Answer
560.4k+ views
Hint:Calcium acetate is a chemical compound which is a calcium salt of acetic acid. Its formula is $Ca({C_2}{H_3}{O_2})$, whereas calcium formate is the calcium salt of formic acid. Its formula is $Ca{(HCOO)_2}$.
Complete answer:
When a mixture of calcium acetate and calcium formate is heated, acetaldehyde is formed.
The equation is as shown:
$Ca{( - OCOC{H_3})_2} + Ca{( - OCOH)_2}\xrightarrow{\Delta }2{H_3}C - CHO + 2CaC{O_3}$
Acetaldehyde is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.
Its boiling point is ${20.2^ \circ }C$ and melting point is $ - {123.5^ \circ }C$.
Hence, option D is correct.
Additional information:
Acetaldehyde is widely used in the manufacturing of perfumes, drugs, acetic acid, flavoring agents, dyes etc. It is toxic when applied externally for prolonged periods. It was first observed in the year $1774$ by the Swedish pharmacist/chemist Carl Wilhelm Scheele. In the whole world, China is the largest consumer of acetaldehyde.
Note:
All ethyl alcohols which are broken down in the human body are first converted to acetaldehyde, and then this acetaldehyde is converted into acetic acid radicals, also known as acetyl radicals. Acetaldehyde is a poison which is a close relative of formaldehyde.
Complete answer:
When a mixture of calcium acetate and calcium formate is heated, acetaldehyde is formed.
The equation is as shown:
$Ca{( - OCOC{H_3})_2} + Ca{( - OCOH)_2}\xrightarrow{\Delta }2{H_3}C - CHO + 2CaC{O_3}$
Acetaldehyde is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.
Its boiling point is ${20.2^ \circ }C$ and melting point is $ - {123.5^ \circ }C$.
Hence, option D is correct.
Additional information:
Acetaldehyde is widely used in the manufacturing of perfumes, drugs, acetic acid, flavoring agents, dyes etc. It is toxic when applied externally for prolonged periods. It was first observed in the year $1774$ by the Swedish pharmacist/chemist Carl Wilhelm Scheele. In the whole world, China is the largest consumer of acetaldehyde.
Note:
All ethyl alcohols which are broken down in the human body are first converted to acetaldehyde, and then this acetaldehyde is converted into acetic acid radicals, also known as acetyl radicals. Acetaldehyde is a poison which is a close relative of formaldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




