
Which category of the synthetic detergents is used in the toothpaste?
A. Anionic
B. Cationic
C. Neutral
D. None of these
Answer
599.1k+ views
Hint: To answer this question you can think of sodium lauryl sulfate, which is a synthetic detergent used in the toothpaste. Now try to find out its nature by the constituents present in it.
Complete step by step answer:
Synthetic detergents are mainly classified into three categories:
(i) Anionic detergents (ii) Cationic detergents and (iii) Non-ionic detergents
Mainly anionic detergents such as sodium or ammonium lauryl sulfate, sodium dodecylbenzene sulfonate, etc. are used in toothpaste to clean the teeth and to provide a foam that helps to carry away the debris. In anionic detergents, the anionic part of the molecule is involved in the cleansing action.
Further, we should also know that sodium lauryl sulfates have significant antibacterial properties and can penetrate as well as dissolve the plaque.
Here, we can see the structure of sodium lauryl sulfate -
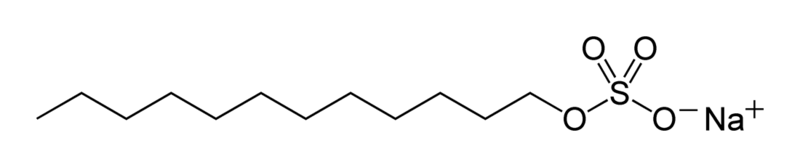
Therefore, we can conclude that the correct answer to this question is option A.
Additional information:
We should also know about other detergents and their uses -
Cationic Detergents - These are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions.
Cetyltrimethylammonium bromide is a popular cationic detergent and is used in hair conditioners.
Non-ionic Detergents - They do not contain any ions in their constitution. One such detergent is formed when stearic acid reacts with polyethylene glycol.
Liquid dishwashing detergents are of non-ionic types.
Note: We should know that these days the branching of the hydrocarbon chain is controlled and kept to the minimum. Unbranched chains can be biodegraded more easily and hence we can prevent pollution.
Complete step by step answer:
Synthetic detergents are mainly classified into three categories:
(i) Anionic detergents (ii) Cationic detergents and (iii) Non-ionic detergents
Mainly anionic detergents such as sodium or ammonium lauryl sulfate, sodium dodecylbenzene sulfonate, etc. are used in toothpaste to clean the teeth and to provide a foam that helps to carry away the debris. In anionic detergents, the anionic part of the molecule is involved in the cleansing action.
Further, we should also know that sodium lauryl sulfates have significant antibacterial properties and can penetrate as well as dissolve the plaque.
Here, we can see the structure of sodium lauryl sulfate -

Therefore, we can conclude that the correct answer to this question is option A.
Additional information:
We should also know about other detergents and their uses -
Cationic Detergents - These are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions.
Cetyltrimethylammonium bromide is a popular cationic detergent and is used in hair conditioners.
Non-ionic Detergents - They do not contain any ions in their constitution. One such detergent is formed when stearic acid reacts with polyethylene glycol.
Liquid dishwashing detergents are of non-ionic types.
Note: We should know that these days the branching of the hydrocarbon chain is controlled and kept to the minimum. Unbranched chains can be biodegraded more easily and hence we can prevent pollution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




