
Which catalyst is used in the preparation of dacron?
Answer
601.8k+ views
Hint: Catalyst is a substance that speeds the rate of a reaction without itself undergoing any chemical change. It participates in the chemical reaction but their chemical properties remain unchanged. Dacron or terylene is the condensation polymerization of ethylene glycol and terephthalic acid. Now we will see the complete step by step solution and identify the catalyst used in the preparation of dacron.
Complete step by step solution:
Dacron is polyester which can be obtained by condensation polymerization of ethylene glycol and terephthalic acid. The reaction takes place as follow:
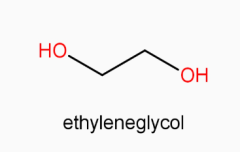 (ethylene glycol) +
(ethylene glycol) +
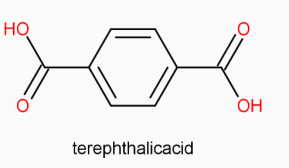 (terephthalic acid) to give
(terephthalic acid) to give
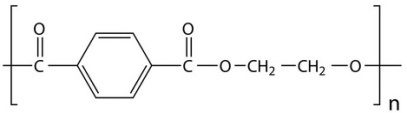 (dacron)
(dacron)
The reaction takes place in the presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide. Catalyst zinc acetate and antimony trioxide is used here to speed up the reaction.
So, we can say that a mixture of zinc acetate and antimony trioxide is used as catalyst in the preparation of dacron.
Additional Information:
- Dacron is known by many names such as in Russia it is called Lavsan, in Britain it is called Terylene.
- Dacron has high tensile strength, high resistance to stretching, and good resistance to degradation by chemical bleaches and to abrasion.
- It is extensively used in the textile industry to make hard wear clothes and dress material.
- Terylene fabric is a synthetic polyester fiber which has properties of terephthalic acid.
- It is resistant to friction.
Note: Mixture of zinc acetate and antimony trioxide is used as a catalyst because it offers high catalytic activity, flame retardation, and allows minimal catalytic side activity.
Complete step by step solution:
Dacron is polyester which can be obtained by condensation polymerization of ethylene glycol and terephthalic acid. The reaction takes place as follow:



The reaction takes place in the presence of a catalyst consisting of a mixture of zinc acetate and antimony trioxide. Catalyst zinc acetate and antimony trioxide is used here to speed up the reaction.
So, we can say that a mixture of zinc acetate and antimony trioxide is used as catalyst in the preparation of dacron.
Additional Information:
- Dacron is known by many names such as in Russia it is called Lavsan, in Britain it is called Terylene.
- Dacron has high tensile strength, high resistance to stretching, and good resistance to degradation by chemical bleaches and to abrasion.
- It is extensively used in the textile industry to make hard wear clothes and dress material.
- Terylene fabric is a synthetic polyester fiber which has properties of terephthalic acid.
- It is resistant to friction.
Note: Mixture of zinc acetate and antimony trioxide is used as a catalyst because it offers high catalytic activity, flame retardation, and allows minimal catalytic side activity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




