
Which are the monomers of Buna-N?
A. Buta-1,3-diene and prop-1-ene-1-nitrile
B. Buta-1,3-diene and acrylonitrile
C. Buta-1,3-diene and prop-2-ene-1-nitrile
D. Buta-1,2-diene and prop-1-ene-1-nitrile
Answer
609.3k+ views
Hint: It is known that Buna-S has Buta-1,3-diene and Styrene as its monomer. The IUPAC name of styrene is ethenylbenzene and both Buna-S and Buna-N are copolymer.
Complete Step by Step Solution:
Try to recall that monomer is the smallest structural unit of a polymer. It is known that Buna-N is a co-polymer which is prepared by the addition polymerization method of monomers Buta-1,3-diene and acrylonitrile. So, in order to find out which option is correct, lets inspect all the above given options.
We should know that prop-1-ene-1-nitrile is the IUPAC name of acrylonitrile and Buta-1,3-diene and acrylonitrile are the monomers of Buna-N. So, option A is the correct answer.
Now, we already know that Buta-1,3-diene and acrylonitrile are the monomers of Buna-N. So, option B is also the correct answer.
The structure of Buta-1,3-diene is:

And, the structure of acrylonitrile or prop-1-ene-1-nitrile is:
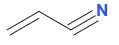
Therefore, it is concluded that both option a and b are correct.
Additional Information:
Copolymer is defined as “a polymer which is formed when two or more different types of polymer are linked with each other in the same polymer chain”. Example- Buna-S which is formed by the copolymerization of monomers recall that monomer is the smallest structural unit of a polymer and Styrene.
Additional polymers are defined as “Those polymers which are formed by simple linking of monomers without formation of any by-product”. Example-Polyethylene which is formed by addition polymerization method having ethylene as its monomer.
Note: Addition polymerization is also called chain growth polymerization.
Buna-N is also known as NBR which stands for Nitrile-Butadiene-Rubber.
Complete Step by Step Solution:
Try to recall that monomer is the smallest structural unit of a polymer. It is known that Buna-N is a co-polymer which is prepared by the addition polymerization method of monomers Buta-1,3-diene and acrylonitrile. So, in order to find out which option is correct, lets inspect all the above given options.
We should know that prop-1-ene-1-nitrile is the IUPAC name of acrylonitrile and Buta-1,3-diene and acrylonitrile are the monomers of Buna-N. So, option A is the correct answer.
Now, we already know that Buta-1,3-diene and acrylonitrile are the monomers of Buna-N. So, option B is also the correct answer.
The structure of Buta-1,3-diene is:

And, the structure of acrylonitrile or prop-1-ene-1-nitrile is:

Therefore, it is concluded that both option a and b are correct.
Additional Information:
Copolymer is defined as “a polymer which is formed when two or more different types of polymer are linked with each other in the same polymer chain”. Example- Buna-S which is formed by the copolymerization of monomers recall that monomer is the smallest structural unit of a polymer and Styrene.
Additional polymers are defined as “Those polymers which are formed by simple linking of monomers without formation of any by-product”. Example-Polyethylene which is formed by addition polymerization method having ethylene as its monomer.
Note: Addition polymerization is also called chain growth polymerization.
Buna-N is also known as NBR which stands for Nitrile-Butadiene-Rubber.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




