
Which among the following molecules can exhibit tautomerism?
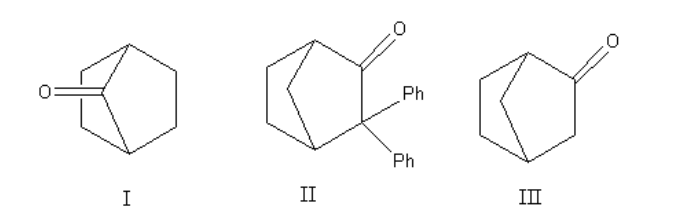
A. Both I and II
B. Both II and III
C. III only
D. Both I and III

Answer
572.1k+ views
Hint:Tautomers are structural isomers of each other. They differed from each other in the position of the proton and electron. Ketone functional group having \[\alpha \] hydrogen undergoes keto-enol tautomerism. All the given molecules are bridge compounds so use Bredt’s rule and determine which molecule undergoes tautomerism.
Complete answer:
Keto-enol tautomerism is the conversion of a ketone into enol form. There is a chemical equilibrium between both forms.
First, we will write the possible enol isomers for all three molecules.
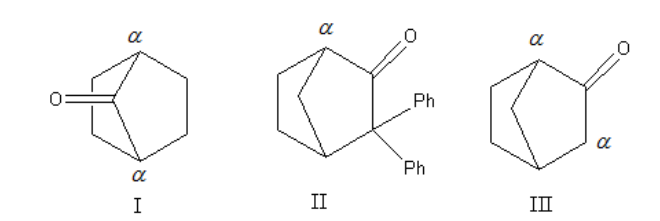
To convert keto form to enol form compound should contain \[\alpha \] hydrogen. So we will assign the \[\alpha \] hydrogen to all three structures.
Now, we will write the tautomerism reaction for all three compounds and will predict the possible products.
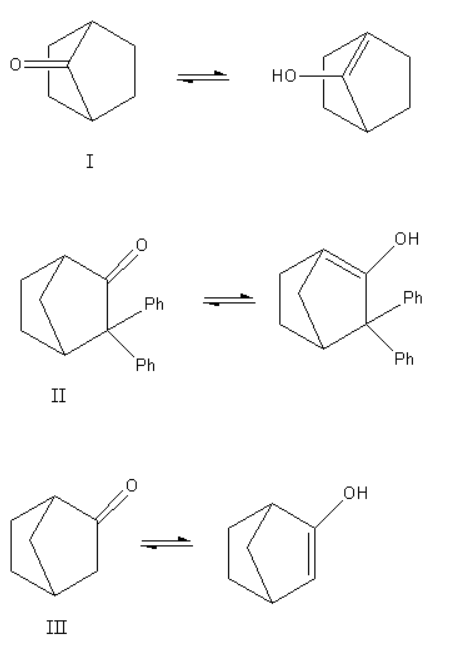
Here, we can see that structure I and II give the bridgehead double bond. According to Bredt’s rule in the case of a bridged ring, the bridgehead double bond does not exist as the bridgehead double bond is unstable. As the enols of structure, I and II have bridgehead double bonds they do not exist. Only structure III gives a non-bridgehead double bond so it shows keto-enol tautomerism.
Thus, out of the three structures only structure III exhibit tautomerism.
So, the correct option is (C) III only.
Note:
The molecule should obey two conditions to undergo tautomerism. The first condition is that the molecule should have \[\alpha \] hydrogen. The second condition is for bridge ring molecules. If the molecule is a bridged ring the double bond should not be bridgehead.
Complete answer:
Keto-enol tautomerism is the conversion of a ketone into enol form. There is a chemical equilibrium between both forms.
First, we will write the possible enol isomers for all three molecules.
To convert keto form to enol form compound should contain \[\alpha \] hydrogen. So we will assign the \[\alpha \] hydrogen to all three structures.

Now, we will write the tautomerism reaction for all three compounds and will predict the possible products.

Here, we can see that structure I and II give the bridgehead double bond. According to Bredt’s rule in the case of a bridged ring, the bridgehead double bond does not exist as the bridgehead double bond is unstable. As the enols of structure, I and II have bridgehead double bonds they do not exist. Only structure III gives a non-bridgehead double bond so it shows keto-enol tautomerism.
Thus, out of the three structures only structure III exhibit tautomerism.
So, the correct option is (C) III only.
Note:
The molecule should obey two conditions to undergo tautomerism. The first condition is that the molecule should have \[\alpha \] hydrogen. The second condition is for bridge ring molecules. If the molecule is a bridged ring the double bond should not be bridgehead.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




