
Which among the following is a non-reducing saccharide?
a) Lactose
b) Maltose
c) Sucrose
d) Fructose
Answer
585.6k+ views
Hint: A sugar/saccharide is called reducing sugar/saccharide; if it has free anomeric carbon. Check which structure has a free anomeric carbon, that option is your answer.
Complete step by step solution:
A reducing sugar is the sugar/saccharide that is capable of acting as a reducing agent. It is because they have a free aldehyde or a free ketone group. All of the monosaccharides are reducing saccharides, along with some disaccharides, and some of the oligosaccharides and a few polysaccharides. The aldehyde functional group allows the sugar to act as a reducing agent
The monosaccharides can be divided into two groups:
The Aldoses, (they have an aldehyde group) and the ketose (they have a ketone group). Ketoses before they can act as reducing sugars, must first tautomerize themselves to form corresponding aldoses.
Disaccharides/polysaccharides are formed from two or more monosaccharides and can be classified as either reducing or nonreducing. Nonreducing disaccharides/ polysaccharides have glycosidic bonds between their anomeric and they cannot convert to an open-chain form with an aldehyde group. They are stuck in their cyclic form. Reducing disaccharide polysaccharides have only few their anomeric carbons involved in the glycosidic bond, while the other is free and can easily convert to an open-chain form with an aldehyde group.
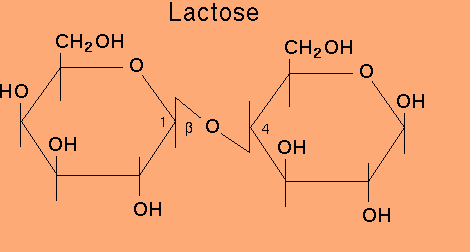
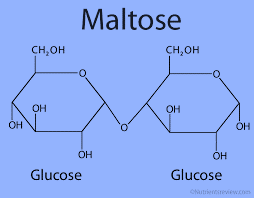
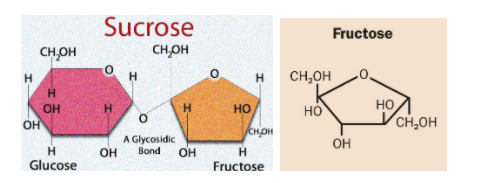
Among the given Sucrose is a non-reducing sugar because anomeric carbon of both the monosaccharides are involved in glycoside or acetal formation. So, it has both is anomeric carbons unavailable to carry out reduction.
So, the correct answer is C)
Note: Ketoses are also reducing sugars, might not be apparent but they can easily tautomerize into aldoses.
Complete step by step solution:
A reducing sugar is the sugar/saccharide that is capable of acting as a reducing agent. It is because they have a free aldehyde or a free ketone group. All of the monosaccharides are reducing saccharides, along with some disaccharides, and some of the oligosaccharides and a few polysaccharides. The aldehyde functional group allows the sugar to act as a reducing agent
The monosaccharides can be divided into two groups:
The Aldoses, (they have an aldehyde group) and the ketose (they have a ketone group). Ketoses before they can act as reducing sugars, must first tautomerize themselves to form corresponding aldoses.
Disaccharides/polysaccharides are formed from two or more monosaccharides and can be classified as either reducing or nonreducing. Nonreducing disaccharides/ polysaccharides have glycosidic bonds between their anomeric and they cannot convert to an open-chain form with an aldehyde group. They are stuck in their cyclic form. Reducing disaccharide polysaccharides have only few their anomeric carbons involved in the glycosidic bond, while the other is free and can easily convert to an open-chain form with an aldehyde group.



Among the given Sucrose is a non-reducing sugar because anomeric carbon of both the monosaccharides are involved in glycoside or acetal formation. So, it has both is anomeric carbons unavailable to carry out reduction.
So, the correct answer is C)
Note: Ketoses are also reducing sugars, might not be apparent but they can easily tautomerize into aldoses.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




