
Which among the following is a cross-linked polymer?
A. Polyesters
B. Glycogens
C. Melamine-formaldehyde
D. Polyvinyl chloride
Answer
557.7k+ views
Hint: Polymer is a large structure having repetition of the same unit. The polymers are of two types, linear and cross-linked. Linear polymers have straight chains. Cross linked polymers have complex structures. We will draw the structure of the repeating unit of the given polymer. Then we will check the polymer having cross linked structure.
Complete step-by-step answer:
Let’s check the structure of polyester.
The alcohol (diol) and acid (dicarboxylic acid) react and form esters. The ester joined in large numbers to form polyester.
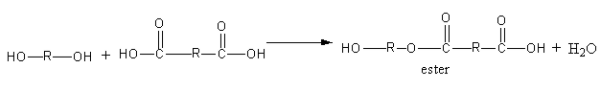
The ester polymerises to give polyester. The repeating unit of polyester is shown as follows:
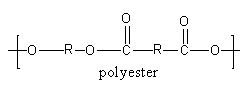
So, polyester has linear structure so, polyester is not a cross linked polymer.
Glycogen is a polymer of glucose. So, the monomer of glycogen is glucose. The glucose units joins by $1,4 - $ glycosidic bond to form a long polymer.
The structure of monomer is as follows:
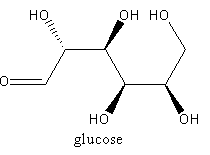
Glycogen is a linear polymer, not cross linked.
Melamine and formaldehyde react to give an intermediate structure which joins in large numbers to give a Melamine-formaldehyde polymer.
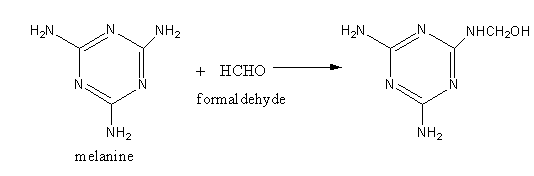
The structure of Melamine-formaldehyde polymer is as follows:
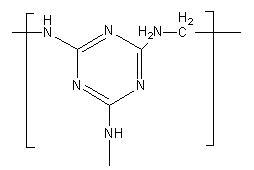
In the reaction of melamine and formaldehyde, the amine group reacts with formaldehyde as melamine has three amine groups. All amine groups can react, so melamine-formaldehyde is a cross linked polymer.
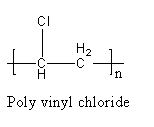
The structure of vinyl chloride is as follows:
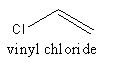
The polymer of PVC is represented as follows:
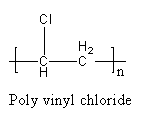
The polymer polyvinyl chloride is formed by the polymerization of polyvinyl chloride and it has a linear structure, so polyvinyl chloride is a linear polymer, not cross linked. The repeating unit or monomer of PVC is vinyl chloride.
The structure of vinyl chloride is as follows:
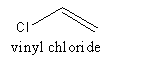
The polymer of PVC is represented as follows:
So, melamine-formaldehyde is a cross-linked polymer.
Therefore, option (C) Melamine-formaldehyde, is correct.
Note: The unit which repeats again and again is known as monomer or repeating unit. Based on the type of polymer units the polymer is divided into two types. When only one type of repeating unit is present in a polymer the polymer is known as a homo-polymer. When more than one type of repeating unit is present in a polymer the polymer is known as copolymer. Polyester and poly vinyl chloride are homo-polymers.
Complete step-by-step answer:
Let’s check the structure of polyester.
The alcohol (diol) and acid (dicarboxylic acid) react and form esters. The ester joined in large numbers to form polyester.

The ester polymerises to give polyester. The repeating unit of polyester is shown as follows:
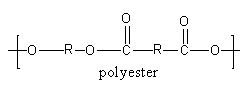
So, polyester has linear structure so, polyester is not a cross linked polymer.
Glycogen is a polymer of glucose. So, the monomer of glycogen is glucose. The glucose units joins by $1,4 - $ glycosidic bond to form a long polymer.
The structure of monomer is as follows:

Glycogen is a linear polymer, not cross linked.
Melamine and formaldehyde react to give an intermediate structure which joins in large numbers to give a Melamine-formaldehyde polymer.

The structure of Melamine-formaldehyde polymer is as follows:

In the reaction of melamine and formaldehyde, the amine group reacts with formaldehyde as melamine has three amine groups. All amine groups can react, so melamine-formaldehyde is a cross linked polymer.
The structure of vinyl chloride is as follows:

The polymer of PVC is represented as follows:

The polymer polyvinyl chloride is formed by the polymerization of polyvinyl chloride and it has a linear structure, so polyvinyl chloride is a linear polymer, not cross linked. The repeating unit or monomer of PVC is vinyl chloride.
The structure of vinyl chloride is as follows:

The polymer of PVC is represented as follows:

So, melamine-formaldehyde is a cross-linked polymer.
Therefore, option (C) Melamine-formaldehyde, is correct.
Note: The unit which repeats again and again is known as monomer or repeating unit. Based on the type of polymer units the polymer is divided into two types. When only one type of repeating unit is present in a polymer the polymer is known as a homo-polymer. When more than one type of repeating unit is present in a polymer the polymer is known as copolymer. Polyester and poly vinyl chloride are homo-polymers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




