
Which among the following are isostructural?
A)$Xe{F_2},I{F_2}^ - $
B)$N{H_3},B{H_3}$
C)$C{O_3}^{2 - }$
D)$PC{l_5},IC{l_5}$
Answer
584.7k+ views
Hint: We know that the isostructural species are the compounds that have the same structure. For finding the isostructural species we have to find the hybridization of every species of the central atom.
Complete step by step answer:
Let us see the structure of species in options,
The Lewis structure of xenon difluoride is,
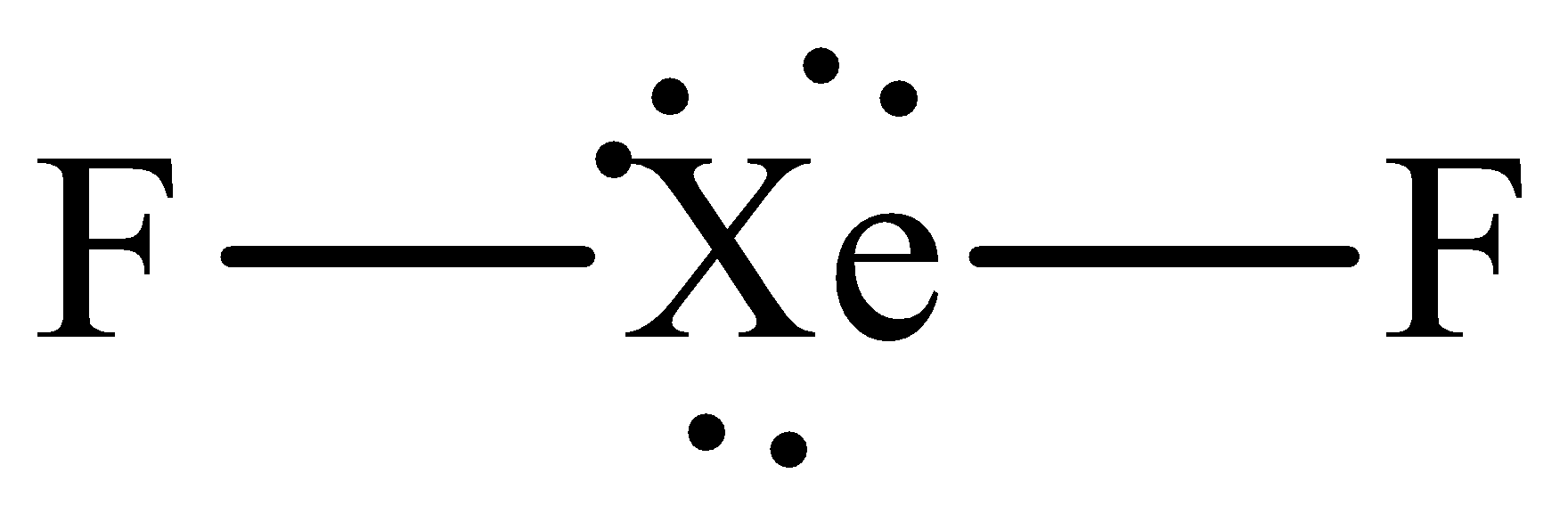
The steric number of central xenon atom is five which means that it is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridized but it has only two bonding electrons thus it adopts the linear geometry with the bond angle of \[{\text{18}}{{\text{0}}^{\text{o}}}{\text{.}}\]
The structure of ${\text{I}}{{\text{F}}_{\text{2}}}^{\text{ - }}$ is,
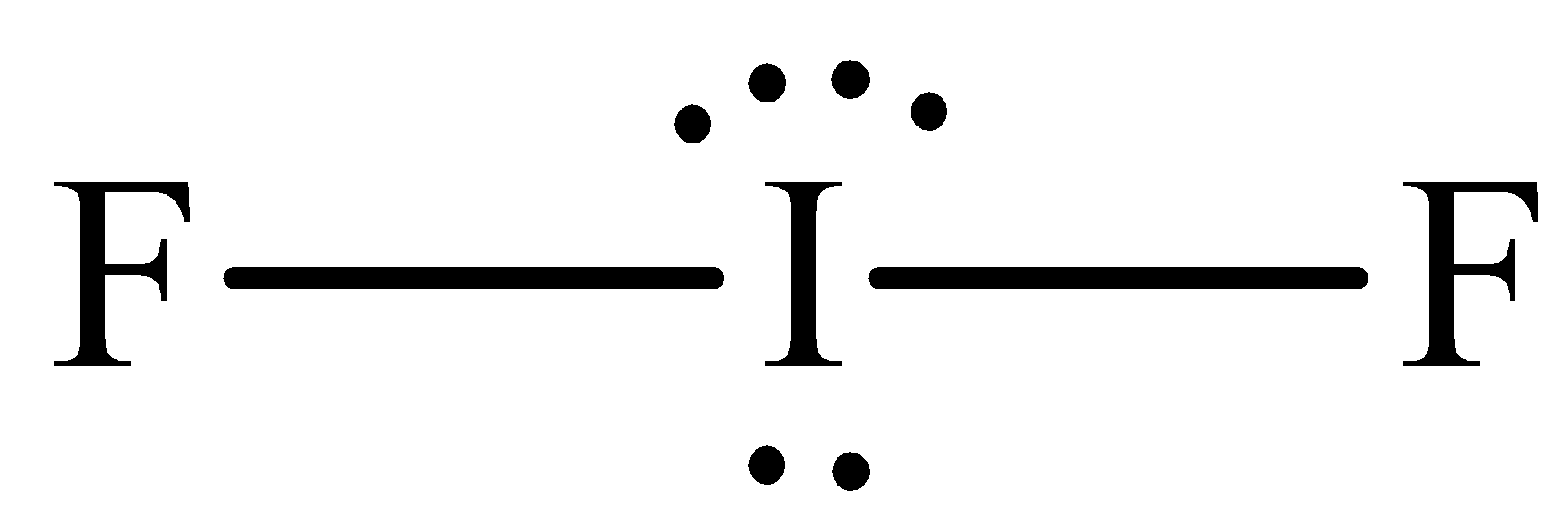
The steric number of ${\text{I}}{{\text{F}}_{\text{2}}}^{\text{ - }}$ is five ,which means that it is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridized, two of them are in bonding and there is lone pairs thus it adopts the linear structure with the bond angle of ${\text{18}}{{\text{0}}^{\text{o}}}$. Hence species in the option A are isostructural.
The structure of $N{H_3}$ is,
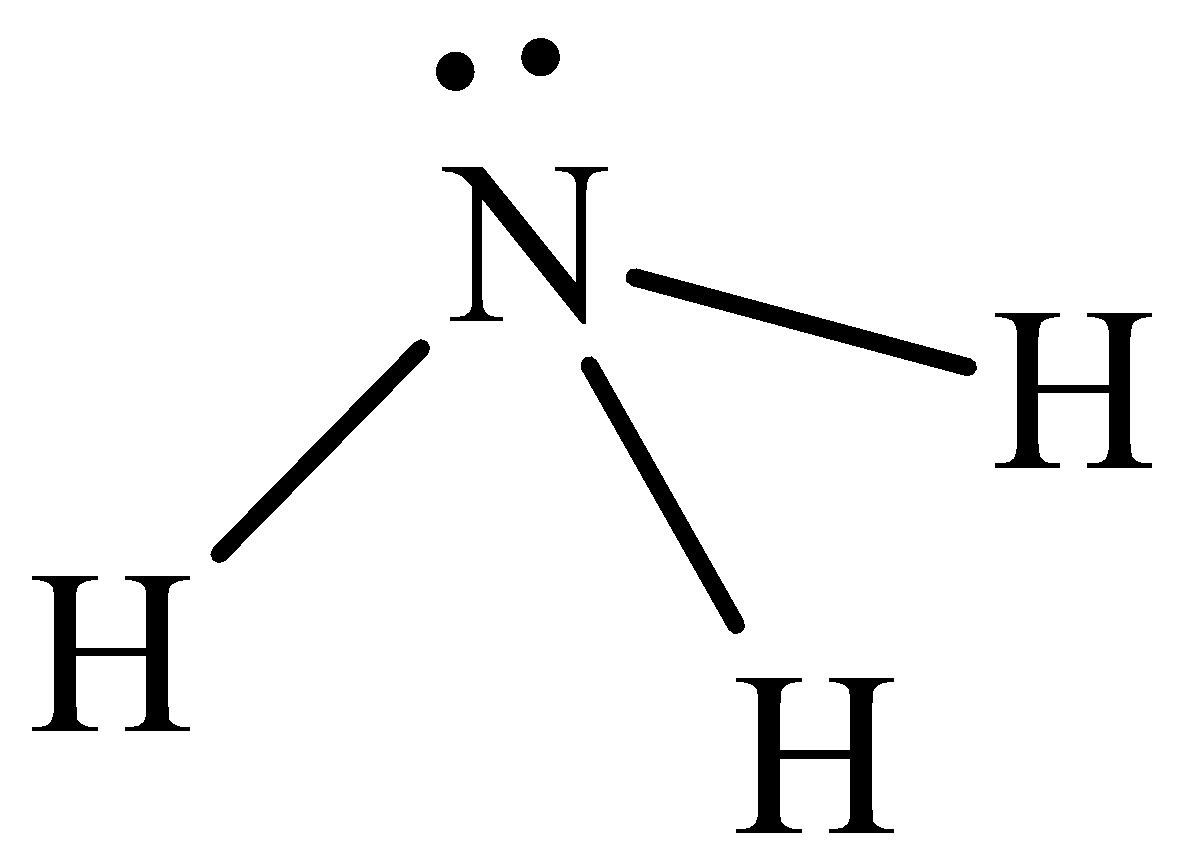
A ${\text{N}}{{\text{H}}_{\text{3}}}$ molecule has three covalent bonds and one lone pair of electrons. Therefore, it is appropriate to use ${\text{s}}{{\text{p}}_{\text{3}}}$ hybrid orbitals on the nitrogen atom. Three of these ${\text{s}}{{\text{p}}_{\text{3}}}$orbitals form localized bond orbitals by combining with the fluorine p orbitals. Thus, the bonding in a ${\text{N}}{{\text{H}}_{\text{3}}}$ molecule in terms of three localized s-bond orbitals and one non bonded lone pair in an ${\text{s}}{{\text{p}}_{\text{3}}}$orbital on the nitrogen atom. There are eight valence electrons in a ${\text{N}}{{\text{H}}_{\text{3}}}$molecule. Six of them occupy the three localized N (${\text{s}}{{\text{p}}_{\text{3}}}$) + (p) s-bond orbitals and two occupy the non bonded N (${\text{s}}{{\text{p}}_{\text{3}}}$) orbital. The use of ${\text{s}}{{\text{p}}_{\text{3}}}$orbitals implies that the H–N–H bond angles are${\text{109}}{\text{.}}{{\text{5}}^{\text{o}}}$
The structure of ${\text{B}}{{\text{H}}_{\text{3}}}$ is,
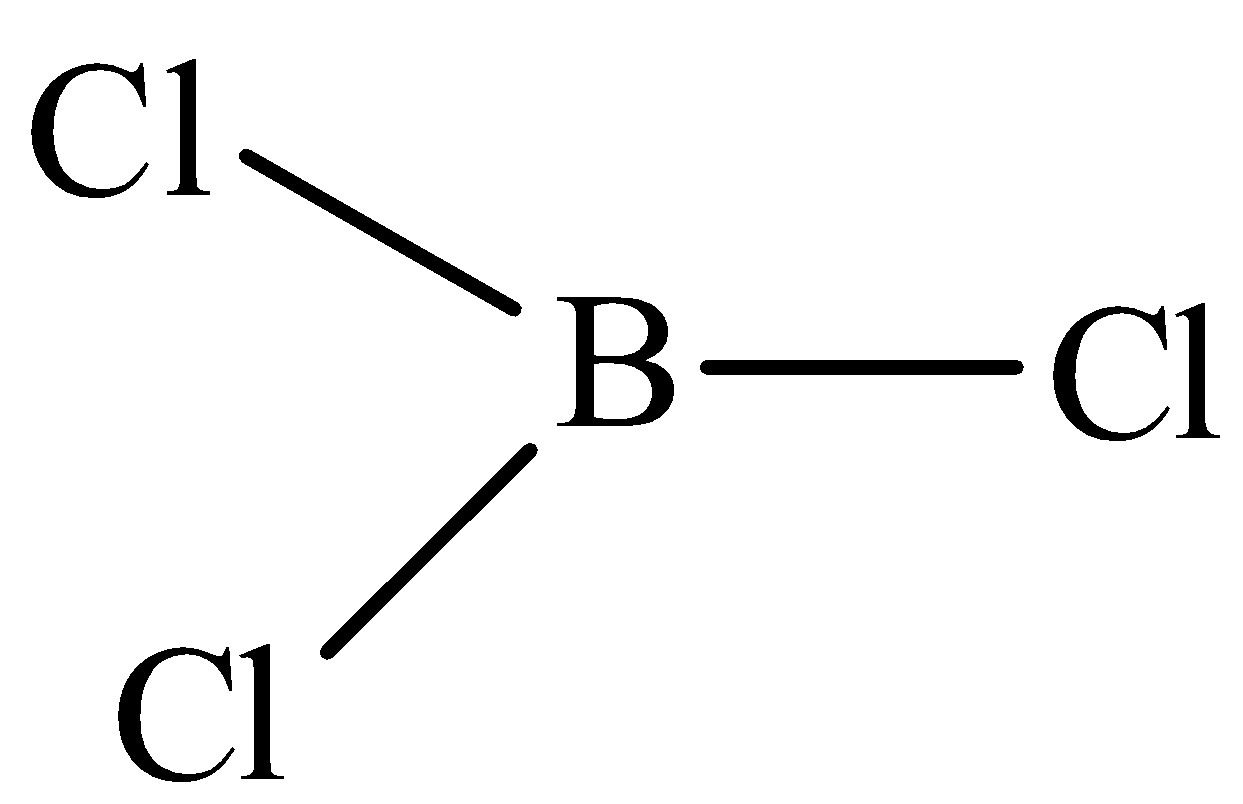
The structure of ${\text{B}}{{\text{H}}_{\text{3}}}$ is trigonal planar with the bond angle of ${\text{12}}{{\text{0}}^{\text{o}}}$.
Hence, species in option B are not isostructural.
The structure of $PC{l_5}$ is,
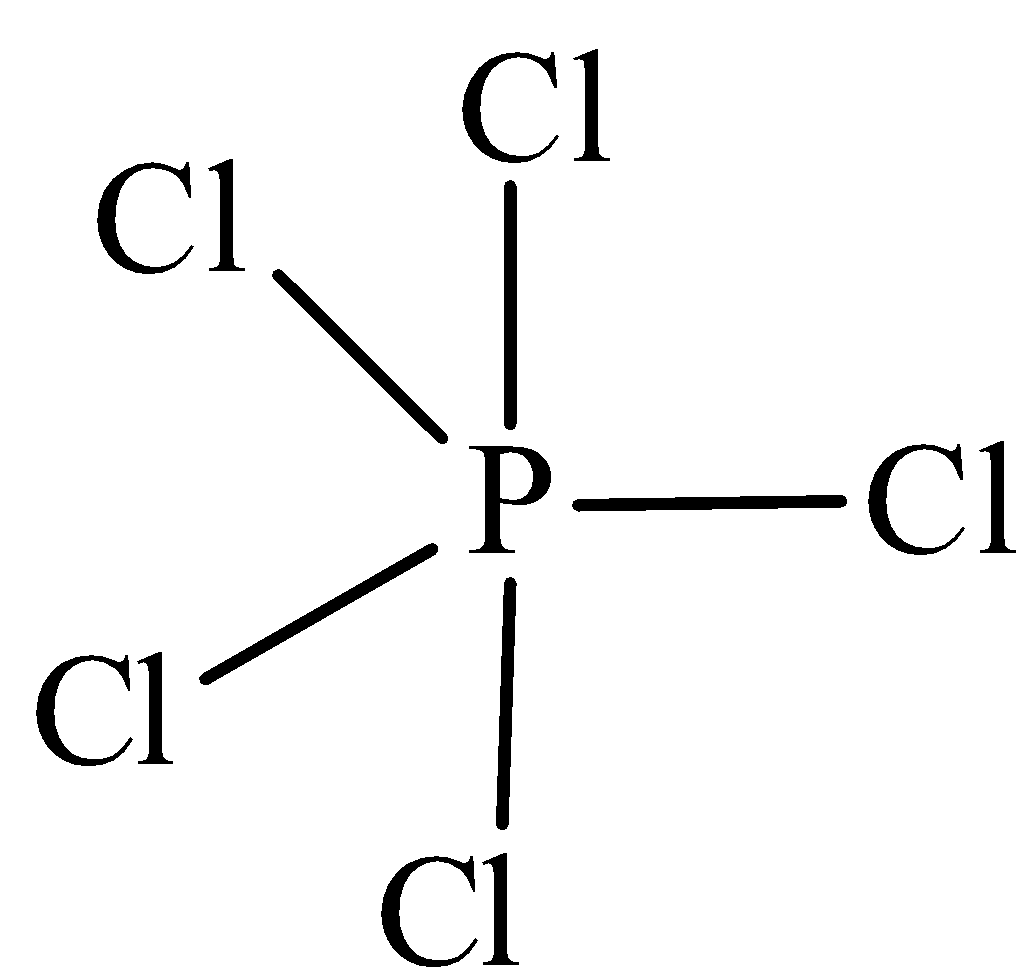
In $PC{l_5}$ the central atom is phosphorus has five bonding domains. The hybridization of $PC{l_5}$ is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$. The molecular geometry is trigonal bipyramidal.
The structure of $IC{l_5}$ is,
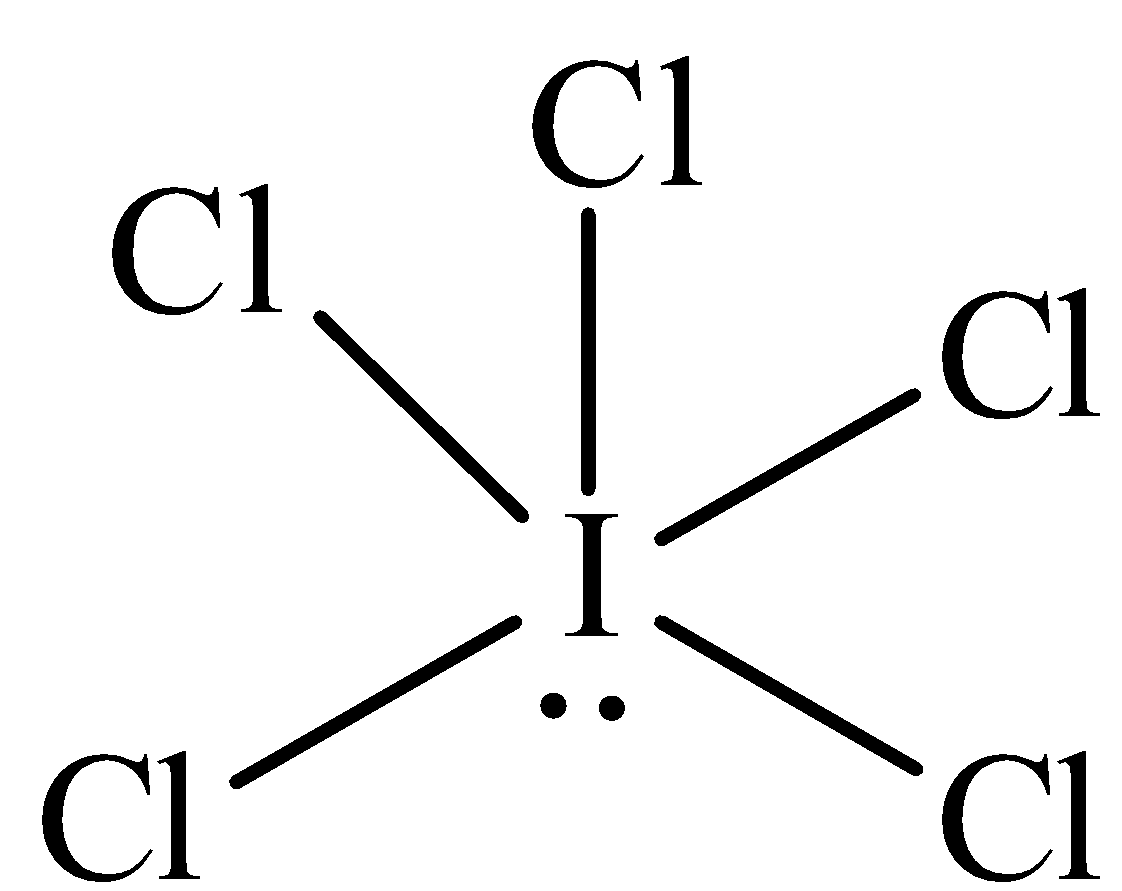
In $IC{l_5}$ the central atom is xenon has one lone pair of electrons and five bonding domains. The hybridization of $IC{l_5}$ is ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}.$The electron pair geometry is octahedral and the molecular geometry is square pyramidal.
Therefore option A is correct.
Note:
We must remember that those species that have the same number of electrons are called isoelectronic species.
Example:
The total number of electrons in ${\text{C}}{{\text{N}}^{\text{ - }}}$ is ten. The total number of electrons in ${{\text{N}}_{\text{2}}}$ is ten. The species ${\text{C}}{{\text{N}}^{\text{ - }}}{\text{,}}{{\text{N}}_{\text{2}}}$ are isoelectronic with each other.
Complete step by step answer:
Let us see the structure of species in options,
The Lewis structure of xenon difluoride is,
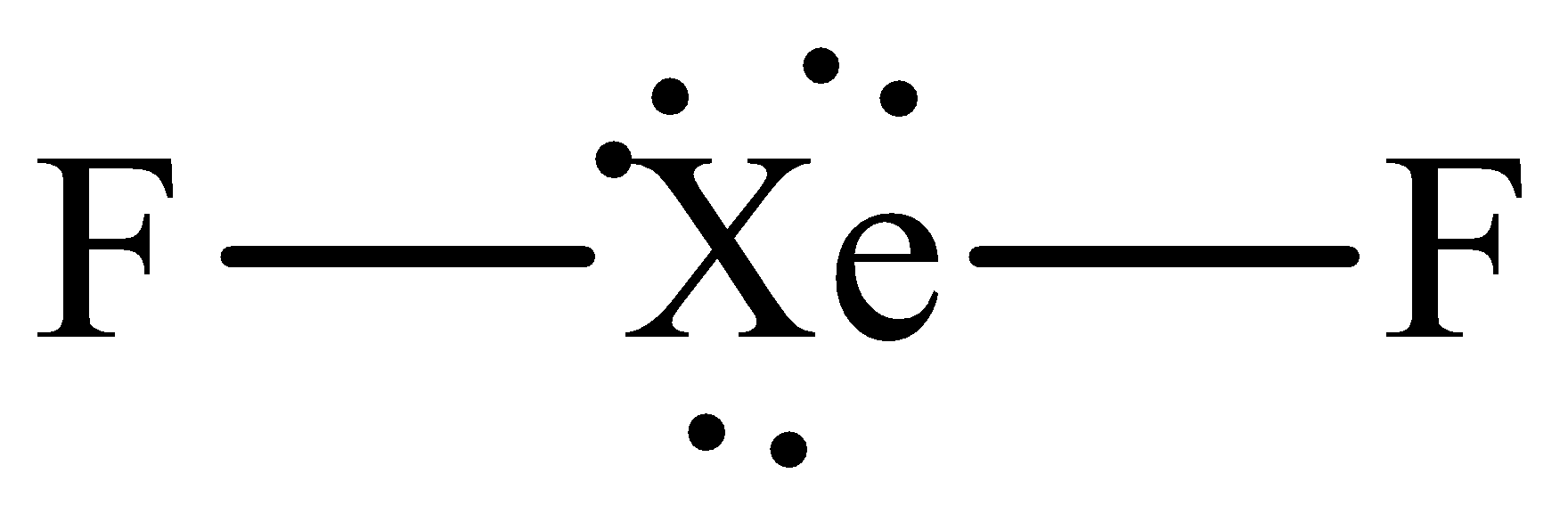
The steric number of central xenon atom is five which means that it is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridized but it has only two bonding electrons thus it adopts the linear geometry with the bond angle of \[{\text{18}}{{\text{0}}^{\text{o}}}{\text{.}}\]
The structure of ${\text{I}}{{\text{F}}_{\text{2}}}^{\text{ - }}$ is,

The steric number of ${\text{I}}{{\text{F}}_{\text{2}}}^{\text{ - }}$ is five ,which means that it is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridized, two of them are in bonding and there is lone pairs thus it adopts the linear structure with the bond angle of ${\text{18}}{{\text{0}}^{\text{o}}}$. Hence species in the option A are isostructural.
The structure of $N{H_3}$ is,

A ${\text{N}}{{\text{H}}_{\text{3}}}$ molecule has three covalent bonds and one lone pair of electrons. Therefore, it is appropriate to use ${\text{s}}{{\text{p}}_{\text{3}}}$ hybrid orbitals on the nitrogen atom. Three of these ${\text{s}}{{\text{p}}_{\text{3}}}$orbitals form localized bond orbitals by combining with the fluorine p orbitals. Thus, the bonding in a ${\text{N}}{{\text{H}}_{\text{3}}}$ molecule in terms of three localized s-bond orbitals and one non bonded lone pair in an ${\text{s}}{{\text{p}}_{\text{3}}}$orbital on the nitrogen atom. There are eight valence electrons in a ${\text{N}}{{\text{H}}_{\text{3}}}$molecule. Six of them occupy the three localized N (${\text{s}}{{\text{p}}_{\text{3}}}$) + (p) s-bond orbitals and two occupy the non bonded N (${\text{s}}{{\text{p}}_{\text{3}}}$) orbital. The use of ${\text{s}}{{\text{p}}_{\text{3}}}$orbitals implies that the H–N–H bond angles are${\text{109}}{\text{.}}{{\text{5}}^{\text{o}}}$
The structure of ${\text{B}}{{\text{H}}_{\text{3}}}$ is,

The structure of ${\text{B}}{{\text{H}}_{\text{3}}}$ is trigonal planar with the bond angle of ${\text{12}}{{\text{0}}^{\text{o}}}$.
Hence, species in option B are not isostructural.
The structure of $PC{l_5}$ is,

In $PC{l_5}$ the central atom is phosphorus has five bonding domains. The hybridization of $PC{l_5}$ is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$. The molecular geometry is trigonal bipyramidal.
The structure of $IC{l_5}$ is,

In $IC{l_5}$ the central atom is xenon has one lone pair of electrons and five bonding domains. The hybridization of $IC{l_5}$ is ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}.$The electron pair geometry is octahedral and the molecular geometry is square pyramidal.
Therefore option A is correct.
Note:
We must remember that those species that have the same number of electrons are called isoelectronic species.
Example:
The total number of electrons in ${\text{C}}{{\text{N}}^{\text{ - }}}$ is ten. The total number of electrons in ${{\text{N}}_{\text{2}}}$ is ten. The species ${\text{C}}{{\text{N}}^{\text{ - }}}{\text{,}}{{\text{N}}_{\text{2}}}$ are isoelectronic with each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




