
What's the geometry of $Be{F_2}$:
A.Linear geometry
B.Bent geometry
C.Tetrahedral geometry
D.Pyramidal geometry
E.Equilateral geometry
Answer
571.8k+ views
Hint: We can calculate the steric number by totaling the number of atoms bonded to the central atom and the lone pairs of electrons present on the central metal atom. Let us know that if the steric number is 4, then we say the atom has $s{p^3}$ hybridization, if the steric number is 3, then atom has $s{p^2}$ hybridization, if the steric number is 2, then it has $sp$ hybridization. We can also predict the geometry of compounds using the steric number.
Complete step by step solution:
As we know that in the compound $Be{F_2}$, the central metal atom is beryllium and two fluorine atoms are attached to beryllium.
The steric number of beryllium atoms can be calculated by adding the number of atoms present in the central metal atom and lone pairs present in the central metal atom.
We can calculate the steric number of beryllium as,
Steric number$ = {\text{No}}{\text{.of atoms bonded with central atom}} + {\text{No}}{\text{.of lone pairs in the central metal atom}}$
And hence,steric number$ = 2 + 0$
Steric number$ = 2$
We have calculated the steric number of beryllium is 2. If an atom has steric number 3, we have to know that hybridization of atoms will be $sp$ and the compound would have linear geometry. The bond angle is ${180^ \circ }$. The geometry of Beryllium fluoride is written as,
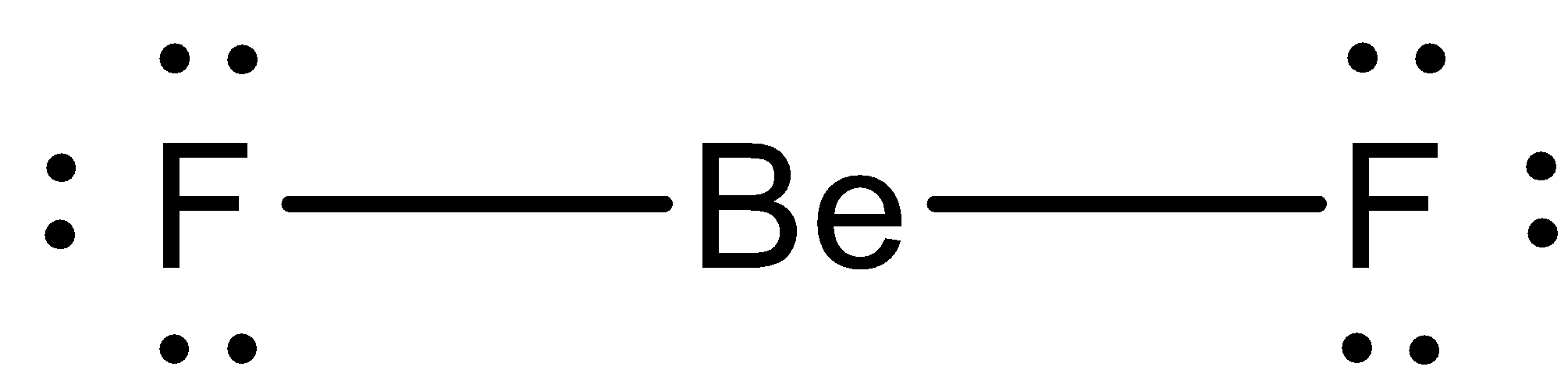
Therefore, the option (A) is correct.
Note:We could also find out the hybridization for organic molecules in the following method,
If an atom has single bonds, then it is $s{p^3}$ hybridized. Example for $s{p^3}$ hybridized molecule is methane. Generally, alkane shows $s{p^3}$ hybridization. Molecules which have $s{p^3}$ hybridization will have tetrahedral geometry.
If an atom has double bonds, then it is $s{p^2}$ hybridized. Example for $s{p^2}$ hybridized molecule is ethene. Generally, alkenes exhibits $s{p^2}$ hybridization. Molecules which have $s{p^2}$ hybridization would have trigonal planar geometry.
If an atom has triple bonds, then it is $sp$ hybridized. Example for $sp$ hybridized molecule is ethyne. Generally, alkynes exhibit $sp$ hybridization.
Complete step by step solution:
As we know that in the compound $Be{F_2}$, the central metal atom is beryllium and two fluorine atoms are attached to beryllium.
The steric number of beryllium atoms can be calculated by adding the number of atoms present in the central metal atom and lone pairs present in the central metal atom.
We can calculate the steric number of beryllium as,
Steric number$ = {\text{No}}{\text{.of atoms bonded with central atom}} + {\text{No}}{\text{.of lone pairs in the central metal atom}}$
And hence,steric number$ = 2 + 0$
Steric number$ = 2$
We have calculated the steric number of beryllium is 2. If an atom has steric number 3, we have to know that hybridization of atoms will be $sp$ and the compound would have linear geometry. The bond angle is ${180^ \circ }$. The geometry of Beryllium fluoride is written as,
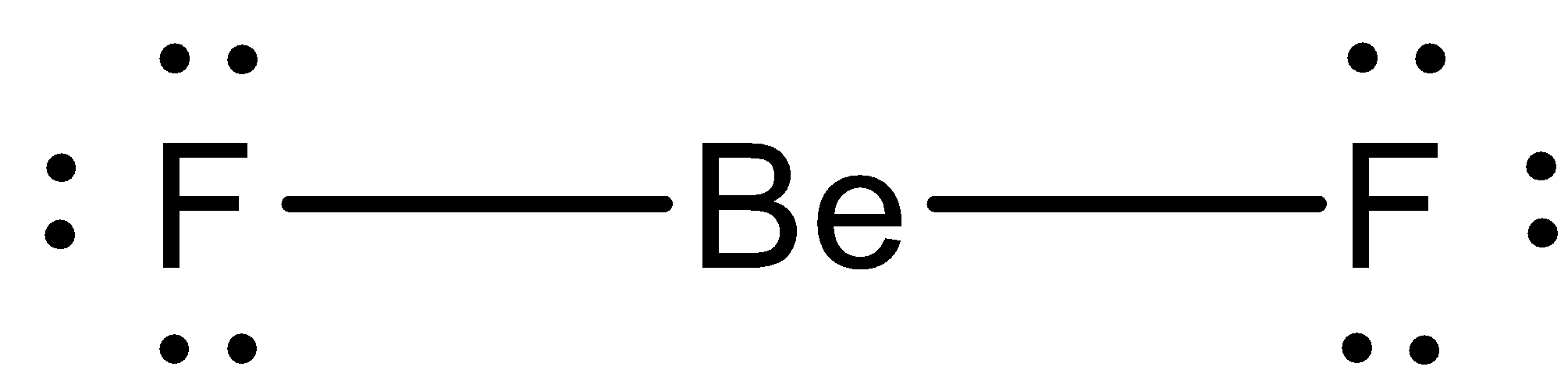
Therefore, the option (A) is correct.
Note:We could also find out the hybridization for organic molecules in the following method,
If an atom has single bonds, then it is $s{p^3}$ hybridized. Example for $s{p^3}$ hybridized molecule is methane. Generally, alkane shows $s{p^3}$ hybridization. Molecules which have $s{p^3}$ hybridization will have tetrahedral geometry.
If an atom has double bonds, then it is $s{p^2}$ hybridized. Example for $s{p^2}$ hybridized molecule is ethene. Generally, alkenes exhibits $s{p^2}$ hybridization. Molecules which have $s{p^2}$ hybridization would have trigonal planar geometry.
If an atom has triple bonds, then it is $sp$ hybridized. Example for $sp$ hybridized molecule is ethyne. Generally, alkynes exhibit $sp$ hybridization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




