
What is W?
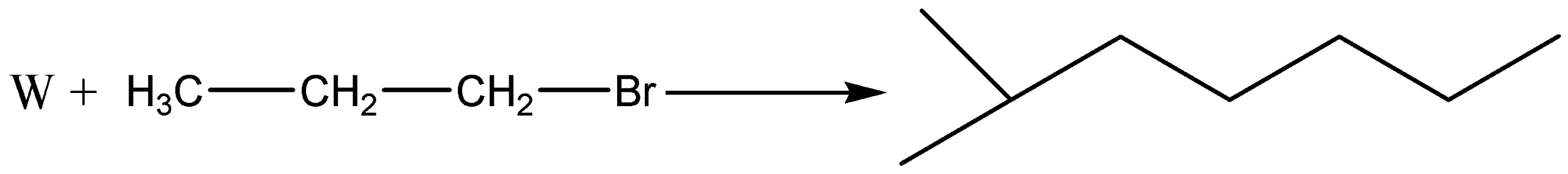
A.
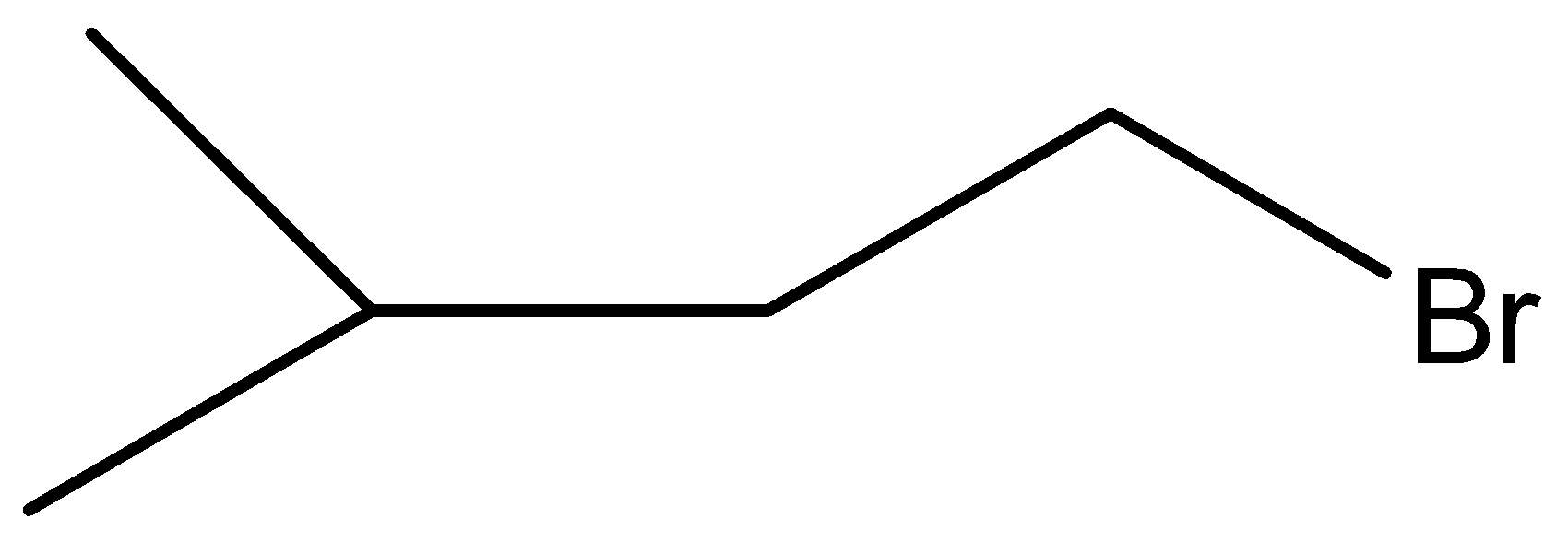
B.
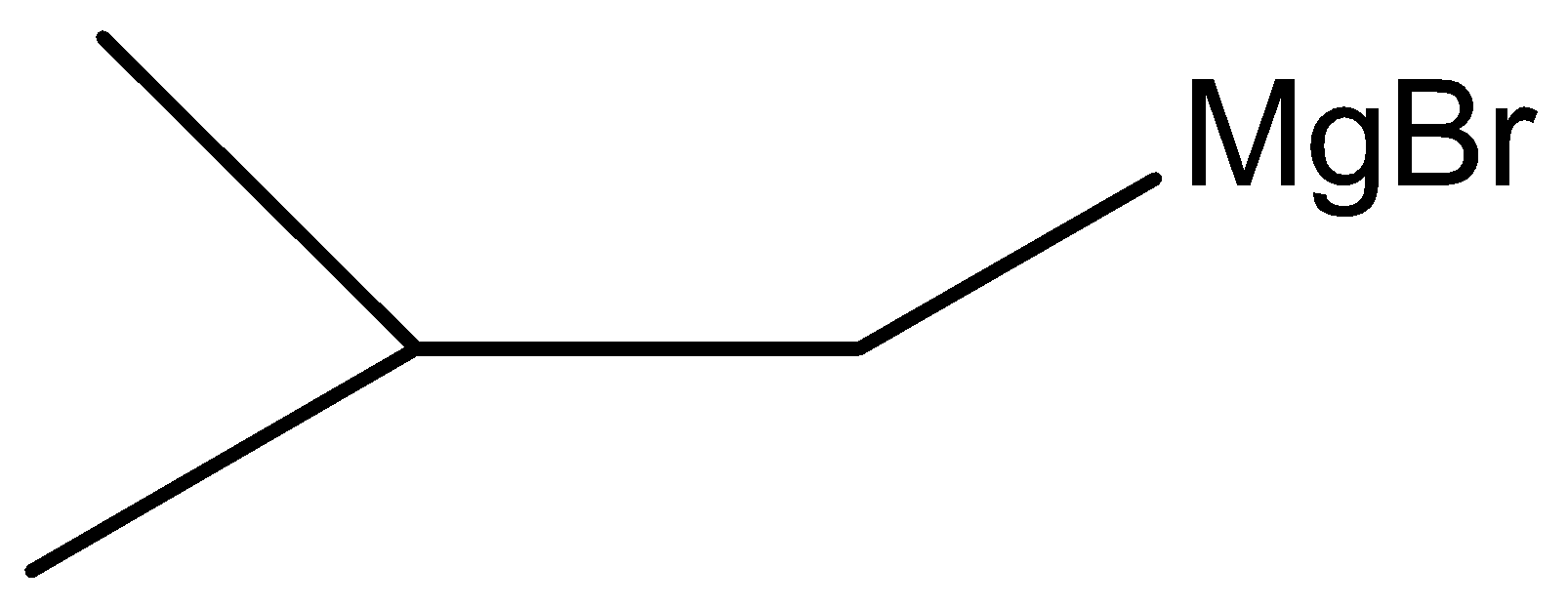
C.
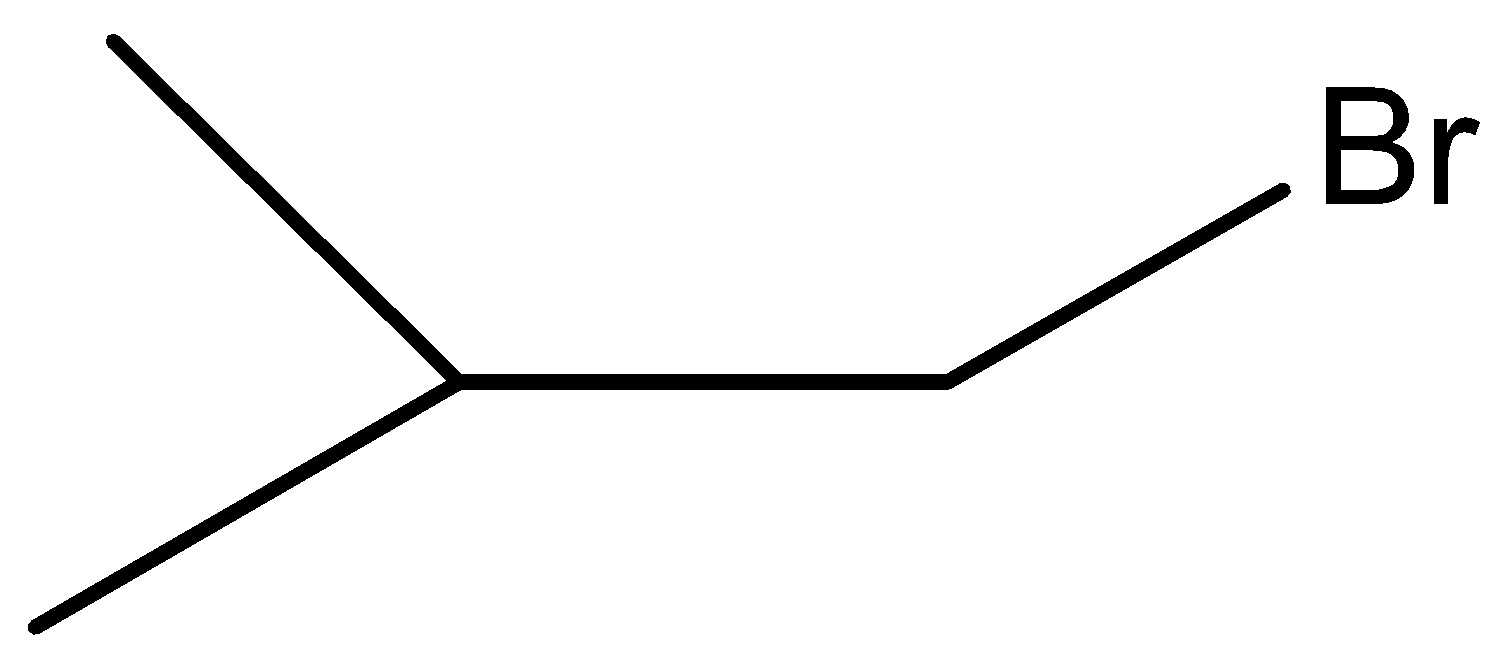
D.
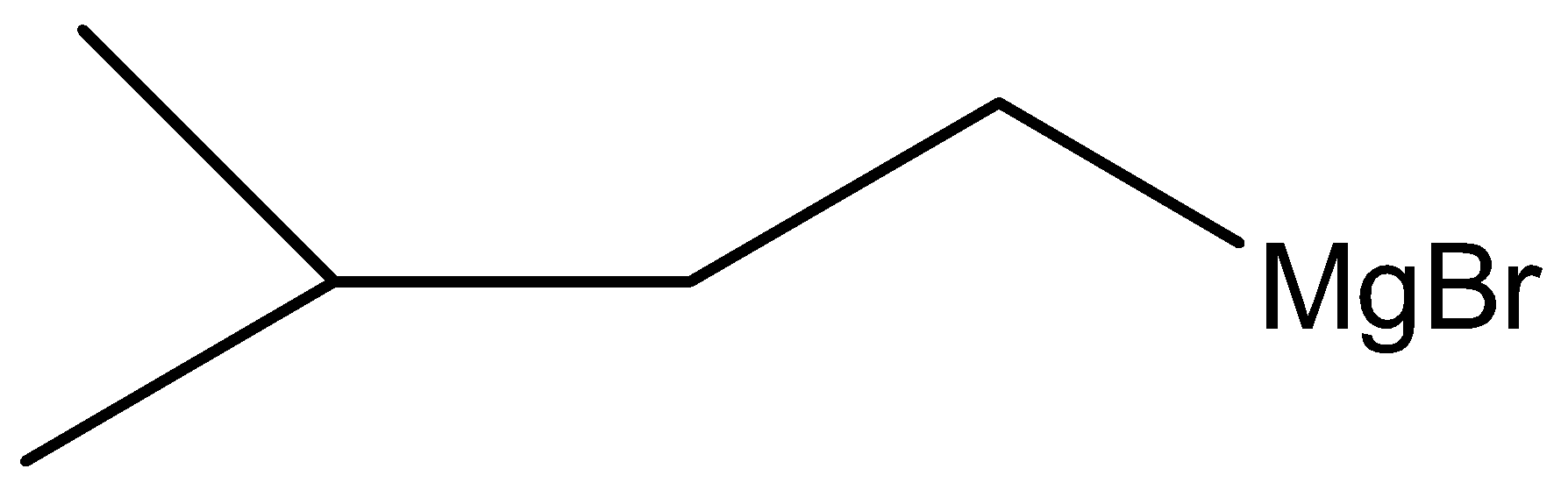





Answer
568.5k+ views
Hint: A Grignard reagent has a formula RMgX where X is a halogen, and R is an alkyl or aryl (based on the benzene ring) group. A typical Grignard reagent might be .
Complete answer:
They can be used for the synthesis of a wide range of organic compounds and are also very useful to the organic chemist.
Grignard reagents are formed by adding the halogenoalkane to a small amount of magnesium in a flask containing ethoxyethane which is commonly called diethyl ether or just ether. The flask is used with a reflux condenser, and the mixture is heated over a water bath for 20 - 30 minutes. The organ magnesium compounds made by the reaction of an alkyl or aryl halide with magnesium are called Grignard reagents. Grignard reagents are used to synthesize a variety of organic compounds and are extremely important to the organic chemist. The highly basic nature of a Grignard reagent usually results in an elimination reaction or no reaction at all. The transition state to add the alkyl halide is less stable than the Magnesium/Bromide(Halide) complex. This is because of a ligation formation between the solvent and the Magnesium atom.
So, the reaction is
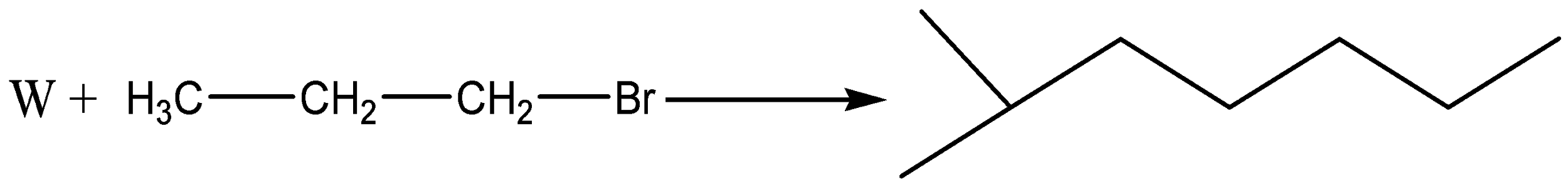
Here, W is
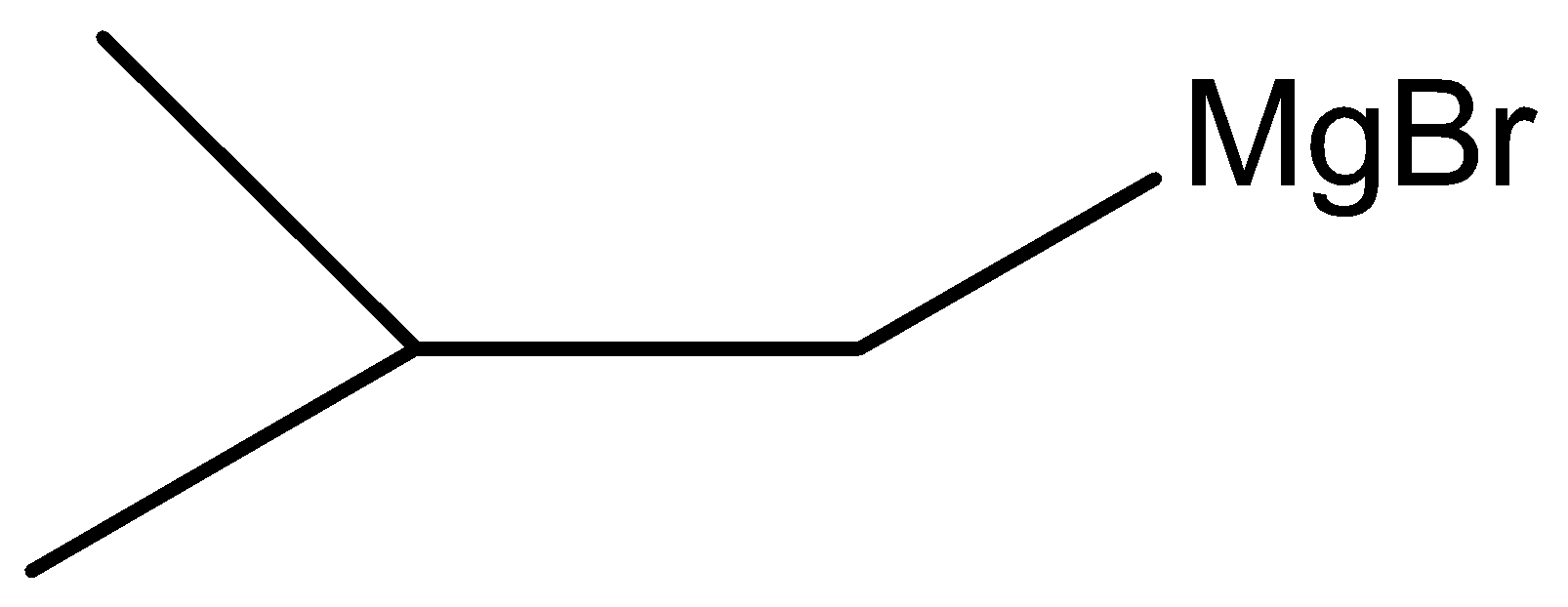
Therefore, the correct answer is option (B).
Note: 1-Bromopropane is a colourless liquid which was originally used in the production of pesticides, flavours, fragrances, pharmaceuticals, and other chemicals. The commercial 1-bromopropane includes not only 1-bromopropane, but also additives that improve its performance in the desired application and stabilizers to inhibit decomposition. It has a role as a neurotoxin and a solvent. It is a bromoalkane and a bromo hydrocarbon.
Complete answer:
They can be used for the synthesis of a wide range of organic compounds and are also very useful to the organic chemist.
Grignard reagents are formed by adding the halogenoalkane to a small amount of magnesium in a flask containing ethoxyethane which is commonly called diethyl ether or just ether. The flask is used with a reflux condenser, and the mixture is heated over a water bath for 20 - 30 minutes. The organ magnesium compounds made by the reaction of an alkyl or aryl halide with magnesium are called Grignard reagents. Grignard reagents are used to synthesize a variety of organic compounds and are extremely important to the organic chemist. The highly basic nature of a Grignard reagent usually results in an elimination reaction or no reaction at all. The transition state to add the alkyl halide is less stable than the Magnesium/Bromide(Halide) complex. This is because of a ligation formation between the solvent and the Magnesium atom.
So, the reaction is

Here, W is

Therefore, the correct answer is option (B).
Note: 1-Bromopropane is a colourless liquid which was originally used in the production of pesticides, flavours, fragrances, pharmaceuticals, and other chemicals. The commercial 1-bromopropane includes not only 1-bromopropane, but also additives that improve its performance in the desired application and stabilizers to inhibit decomposition. It has a role as a neurotoxin and a solvent. It is a bromoalkane and a bromo hydrocarbon.
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




