
What is the valency of Boron in $B{{F}_{3}}$ ?
Answer
513.6k+ views
Hint: We know that valency is the combining power of an atom. The valency of an atom signifies the no. of electrons it can lose, gain or share. The $B{{F}_{3}}$ molecules are covalent in nature. To check the valency of the compound check the valency of each atom and then solve it for the compound.
Complete answer: As we know that valency: The combining capacity of an atom is known as its valency. The number of bonds that an atom can form as part of a compound is expressed by the valency of the element. Valency is a measure of the ability of an atom to bond with other atoms. As we know, the number of electrons in the outermost shell of sodium is one, and in chlorine, it is seven. Therefore the valency of sodium is one as it can easily lose one electron and become stable. On the other hand, chlorine is also one as it can accept one electron easily and also attain stability Furthermore, it is not only determined when an atom loses an electron. For example, chlorine has seven electrons in its outermost orbital. It is hard to lose seven electrons and so it completes its octet by gaining one electron. Since it gains one electron, its valency is one. In the periodic table, the elements in the same group have the same valency.
Boron atomic number is five. So by applying octet rule we get: In K shell two electrons; L shell three electrons as in outer shells it has three electrons which is less than five, so it has to release or donate these electrons for stability. So valency of Boron is three.
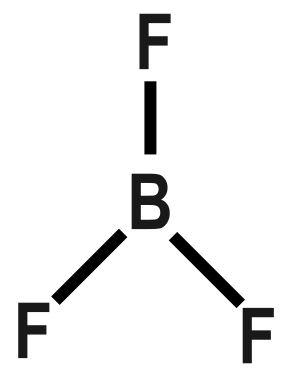
Therefore, it makes three sigma bonds with three fluorine atoms. It has no double bonds. It has a trigonal planar geometry, with Boron at the centre and fluorine bond to it on three sides.
Additional information: Noble gases have a completely filled outermost shell and that’s why they are least reactive. Other element’s reactivity depends upon their ability to attain the noble gas configuration. If the outermost shell has 8 electrons then the element is said to have a complete octet. By gaining, sharing and losing the electrons the atoms complete their outermost orbital and make an octet.
Note:
Remember that the difference between Valency and Oxidation Number: Valency is different from the oxidation number, and it has no sign also the number of bonds an atom makes is its valency at times. Boron exhibits a valency of both three and five. But to gain five electrons is not feasible so it exhibits a valency of three. Make sure that at different times different valences are used.
Complete answer: As we know that valency: The combining capacity of an atom is known as its valency. The number of bonds that an atom can form as part of a compound is expressed by the valency of the element. Valency is a measure of the ability of an atom to bond with other atoms. As we know, the number of electrons in the outermost shell of sodium is one, and in chlorine, it is seven. Therefore the valency of sodium is one as it can easily lose one electron and become stable. On the other hand, chlorine is also one as it can accept one electron easily and also attain stability Furthermore, it is not only determined when an atom loses an electron. For example, chlorine has seven electrons in its outermost orbital. It is hard to lose seven electrons and so it completes its octet by gaining one electron. Since it gains one electron, its valency is one. In the periodic table, the elements in the same group have the same valency.
Boron atomic number is five. So by applying octet rule we get: In K shell two electrons; L shell three electrons as in outer shells it has three electrons which is less than five, so it has to release or donate these electrons for stability. So valency of Boron is three.

Therefore, it makes three sigma bonds with three fluorine atoms. It has no double bonds. It has a trigonal planar geometry, with Boron at the centre and fluorine bond to it on three sides.
Additional information: Noble gases have a completely filled outermost shell and that’s why they are least reactive. Other element’s reactivity depends upon their ability to attain the noble gas configuration. If the outermost shell has 8 electrons then the element is said to have a complete octet. By gaining, sharing and losing the electrons the atoms complete their outermost orbital and make an octet.
Note:
Remember that the difference between Valency and Oxidation Number: Valency is different from the oxidation number, and it has no sign also the number of bonds an atom makes is its valency at times. Boron exhibits a valency of both three and five. But to gain five electrons is not feasible so it exhibits a valency of three. Make sure that at different times different valences are used.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




