
What is the origin of spectral lines?
Answer
573.9k+ views
Hint: Spectral series are the set of wavelengths arranged in a systematic and sequential order or fashion. The spectral lines are also divided into different series like Lyman, Balmer and paschal etc. where each series consists of different wavelengths.
Complete step by step answer:
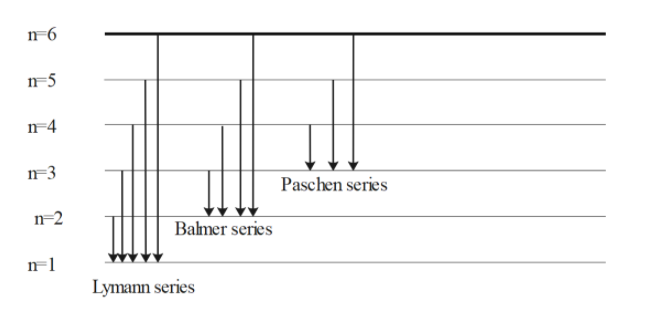
Hydrogen atom is the simplest atomic system found in nature; thus, it produces the simplest of these series. When the beam of light or any radiation is made to enter the device through a slit, each individual component of the light or radiation form images of the source. These images can be visualised when resolved under the spectroscope. The images will be in the form of parallel lines arranged next to each other with regular spacing. The lines will be apart in the higher wavelength side and they come closer gradually when moved from higher to lower wavelength side. The shortest wavelength will possess least spaced spectral lines and it is named as series limit. Atomic hydrogen shows the emission spectrum. This spectrum consists of several spectral lines. When the electrons in the gas get sufficient energy, they make transitions between the energy levels. We can also calculate the wavelength of any spectral line if the atomic number, lower energy level and the upper energy level is given to us. The formula used to find the wavelength of any spectral series is known as Rydberg’s formula. Below is the diagram related to spectral lines.
Note:
In the above information, we need to note that the Rydberg’s constant is valid for hydrogen and hydrogen-like elements. The equations return meaningful value only when the electrons in higher or upper energy levels are more than the electrons present in the lower energy level.
Complete step by step answer:
Hydrogen atom is the simplest atomic system found in nature; thus, it produces the simplest of these series. When the beam of light or any radiation is made to enter the device through a slit, each individual component of the light or radiation form images of the source. These images can be visualised when resolved under the spectroscope. The images will be in the form of parallel lines arranged next to each other with regular spacing. The lines will be apart in the higher wavelength side and they come closer gradually when moved from higher to lower wavelength side. The shortest wavelength will possess least spaced spectral lines and it is named as series limit. Atomic hydrogen shows the emission spectrum. This spectrum consists of several spectral lines. When the electrons in the gas get sufficient energy, they make transitions between the energy levels. We can also calculate the wavelength of any spectral line if the atomic number, lower energy level and the upper energy level is given to us. The formula used to find the wavelength of any spectral series is known as Rydberg’s formula. Below is the diagram related to spectral lines.

Note:
In the above information, we need to note that the Rydberg’s constant is valid for hydrogen and hydrogen-like elements. The equations return meaningful value only when the electrons in higher or upper energy levels are more than the electrons present in the lower energy level.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




