
What is the octane number?
Answer
573.9k+ views
Hint: octane number of gasoline is a measure of the resistance of fuel towards knocking. It is tested by using a mixture of isooctane and n-heptane. Branching hydrocarbons have a greater octane number and straight-line hydrocarbons have low octane numbers. The fuel which has a high octane number gives a good performance.
Complete Solution :
- Octane scale or octane number is used to indicate the quality of fuel. This measures the ability of the fuel to burn in a combustion engine without getting knocked. Knocking is a process by which fuel and air mixture ignites without spark. Knocking may be caused due to defects in the engine or fast-burning fuel. This gasoline air mixture when ignited at the wrong point reduces the power generated by the output and thus further damages the combustion engine. Therefore these gasoline formulations should be designed in such a way that they reduce the chances of knocking and increase engine performance.
- This is how the octane scale or octane number came into the picture. The scale was developed using two hydrocarbons: n-heptane and isooctane. Isooctane has a given number 100 on the octane scale. It is a branched compound and burns smoothly and it has little knocking power. However on the other hand n-heptane is a straight-chain compound and has bad knocking power. It is given number 0(zero) on the octane scale.
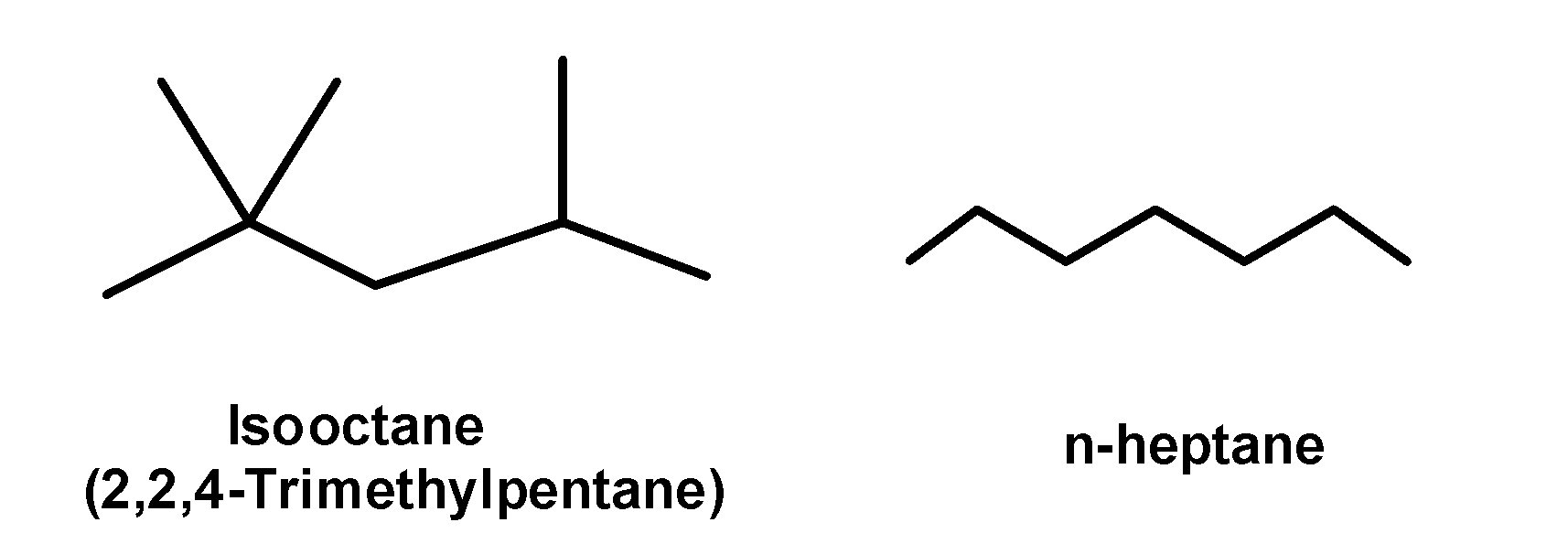
- It is found that the octane number is inversely related to the carbon chain length. It decreases with the increase in the carbon chain. Octane number increases with a decrease in straight chain structure or increases in the branching of hydrocarbon. Octane number is greater for the aromatic compound having the same number of carbons.
Thus, it is advised to purchase gasoline which has a higher octane number. Most of the automobiles use gasoline of the lowest grade of 87 octane.
Note: Note that, greater is the octane number better is the performance of the engine. Octane number is increasing by the isomerization, refining process of gasoline up to 90 numbers. Anti-knocking agents for example tetraethyl lead reduces the internal combustion of the engine and further increases the octane number of fuel.
Complete Solution :
- Octane scale or octane number is used to indicate the quality of fuel. This measures the ability of the fuel to burn in a combustion engine without getting knocked. Knocking is a process by which fuel and air mixture ignites without spark. Knocking may be caused due to defects in the engine or fast-burning fuel. This gasoline air mixture when ignited at the wrong point reduces the power generated by the output and thus further damages the combustion engine. Therefore these gasoline formulations should be designed in such a way that they reduce the chances of knocking and increase engine performance.
- This is how the octane scale or octane number came into the picture. The scale was developed using two hydrocarbons: n-heptane and isooctane. Isooctane has a given number 100 on the octane scale. It is a branched compound and burns smoothly and it has little knocking power. However on the other hand n-heptane is a straight-chain compound and has bad knocking power. It is given number 0(zero) on the octane scale.
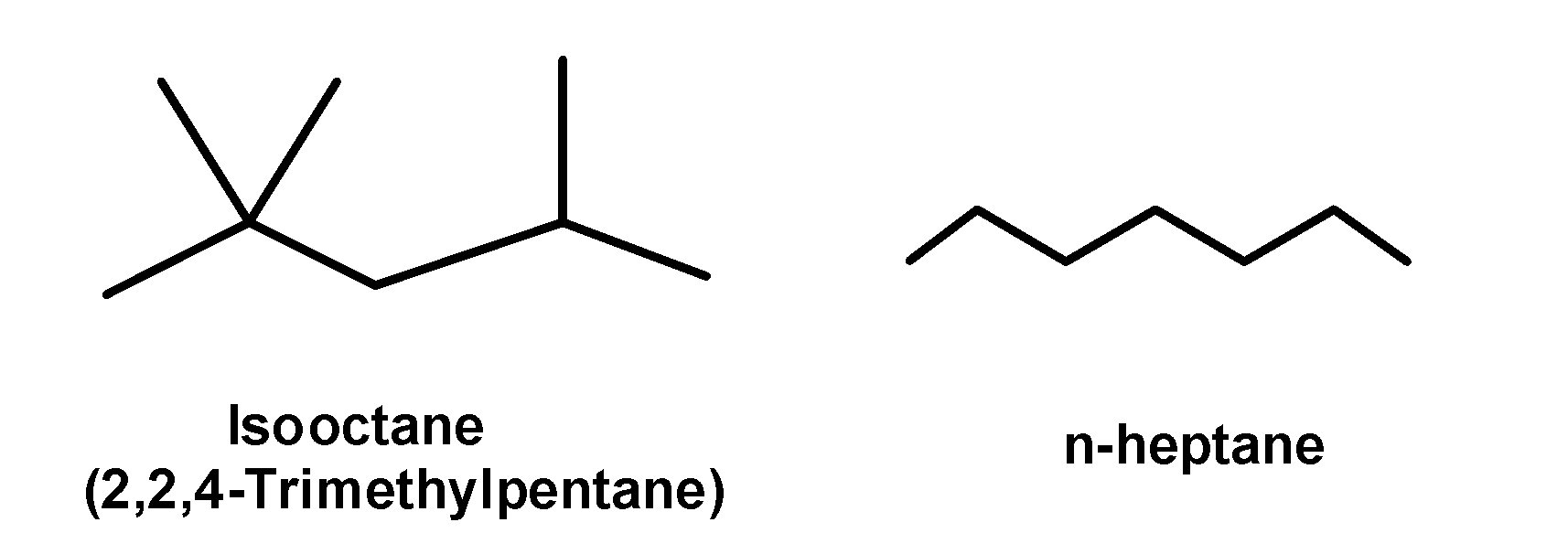
- It is found that the octane number is inversely related to the carbon chain length. It decreases with the increase in the carbon chain. Octane number increases with a decrease in straight chain structure or increases in the branching of hydrocarbon. Octane number is greater for the aromatic compound having the same number of carbons.
Thus, it is advised to purchase gasoline which has a higher octane number. Most of the automobiles use gasoline of the lowest grade of 87 octane.
Note: Note that, greater is the octane number better is the performance of the engine. Octane number is increasing by the isomerization, refining process of gasoline up to 90 numbers. Anti-knocking agents for example tetraethyl lead reduces the internal combustion of the engine and further increases the octane number of fuel.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




