
What is the name for $ K{C_2}{H_3}{O_2} $ ?
Answer
494.4k+ views
Hint: Potassium acetate exists as a crystalline solid which is mild soluble in water as solvent. When we apply heat on the potassium acetate then it will undergo the process of thermal decomposition to form potassium oxides.
Complete answer:
The compound $ K{C_2}{H_3}{O_2} $ contains basically three types of atoms including potassium, oxygen, carbon and hydrogen. As we know, the chemical formula of acetate which is formed by removal of hydrogen atoms from acetic acid is $ {C_2}{H_3}{O_2} $ . When we add potassium with acetate ion it is collectively known as potassium acetate.
When we treat the acetic acid solution with the potassium hydroxide solution then they react with each other to form a salt of potassium which is commonly known as potassium acetate.
\[C{H_3}COOH{\text{ }} + {\text{ }}KOH \to C{H_3}COOH + {H_2}O\]
Hence, we can consider potassium oxide as potassium salt or acetic acid. Potassium acetate is also prepared by treating acetic acid with potassium carbonate.
Potassium acetate exists as a hygroscopic solid under the normal room temperature because it has a tendency to absorb the moisture from the atmosphere.
Chemical structure of potassium acetate is –
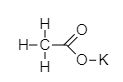
Hence, the name for $ K{C_2}{H_3}{O_2} $ is potassium acetate.
Note:
Potassium acetate is widely used as a source of potassium which is administered to treat the medical condition known as hypokalemia which is associated with lower blood level of potassium in the body. Potassium acetate is known by some other names such as potassium ethanoate or acetic acid potassium salt.
Complete answer:
The compound $ K{C_2}{H_3}{O_2} $ contains basically three types of atoms including potassium, oxygen, carbon and hydrogen. As we know, the chemical formula of acetate which is formed by removal of hydrogen atoms from acetic acid is $ {C_2}{H_3}{O_2} $ . When we add potassium with acetate ion it is collectively known as potassium acetate.
When we treat the acetic acid solution with the potassium hydroxide solution then they react with each other to form a salt of potassium which is commonly known as potassium acetate.
\[C{H_3}COOH{\text{ }} + {\text{ }}KOH \to C{H_3}COOH + {H_2}O\]
Hence, we can consider potassium oxide as potassium salt or acetic acid. Potassium acetate is also prepared by treating acetic acid with potassium carbonate.
Potassium acetate exists as a hygroscopic solid under the normal room temperature because it has a tendency to absorb the moisture from the atmosphere.
Chemical structure of potassium acetate is –

Hence, the name for $ K{C_2}{H_3}{O_2} $ is potassium acetate.
Note:
Potassium acetate is widely used as a source of potassium which is administered to treat the medical condition known as hypokalemia which is associated with lower blood level of potassium in the body. Potassium acetate is known by some other names such as potassium ethanoate or acetic acid potassium salt.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




