
What is the Lewis structure of \[S{{O}_{2}}\]?
Answer
492.9k+ views
Hint: Covalent bond is formed by the sharing of electrons between the two atoms. The octet rule illustrates that the two atoms combine to each other either by transfer of electrons or by sharing of electrons to have an octet (8 electrons) in their Valence shells. Both \[S\] and \[O\] have six electrons in their valence shell.
Complete answer:
In order to draw the Lewis structure of \[S{{O}_{2}}\], first we have to calculate the total number of valence electrons in \[S{{O}_{2}}\] molecule.
\[Total\;no.\;of\;valence\;electrons\\
=valence\;electrons\;of\;S+2\times (valence\;electrons\;of\;O)\]
\[Total\;no.\;of\;valence\;electrons=6+2\times (6)\]
\[Total\;no.\;of\;valence\;electrons=18\]
Herein \[S\] is a central atom and oxygen can make a maximum of two covalent bonds (because of the absence of d-orbitals). Therefore, the structure will be
$ O=S=O$
Now, in order to complete their respective octets, sulfur should have one nonbonding pair of electrons and each oxygen atom should have two nonbonding pairs of electrons. Therefore, the Lewis structure of \[S{{O}_{2}}\] molecule is
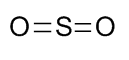
For calculating the formal charge on each atom i.e. \[S\] and \[O\], we have to use the following formula:
\[formal\;charge=valence\;electrons-non\;bonding\;electrons-\dfrac{bonding\;electrons}{2}\]
Therefore, \[formal\; charge\;on\;S=6-2-\dfrac{8}{2}=0\]
\[formal\;charge\;on\;O=6-4-\dfrac{4}{2}=0\]
Additional information: There are two types of bonds which include covalent and ionic bonds. An ionic bond is formed by the transfer of electrons i.e. one atom loses the electrons and another atom accepts the electron. For example: \[NaCl\]. On the other hand, covalent bonds are formed by sharing of electrons. Apart from these, there is one more type of bond which is called the coordinate bond in which sharing of electrons takes place between two atoms but both electrons are given by one atom.
Note:
It is important to note that the Lewis structure of \[S{{O}_{2}}\] contains four covalent bonds. In order to complete their octets, sulfur has one non-bonding pair of electrons while each oxygen has two nonbonding pairs of electrons. The formal charge on each atom i.e. sulfur and oxygen is zero.
Complete answer:
In order to draw the Lewis structure of \[S{{O}_{2}}\], first we have to calculate the total number of valence electrons in \[S{{O}_{2}}\] molecule.
\[Total\;no.\;of\;valence\;electrons\\
=valence\;electrons\;of\;S+2\times (valence\;electrons\;of\;O)\]
\[Total\;no.\;of\;valence\;electrons=6+2\times (6)\]
\[Total\;no.\;of\;valence\;electrons=18\]
Herein \[S\] is a central atom and oxygen can make a maximum of two covalent bonds (because of the absence of d-orbitals). Therefore, the structure will be
$ O=S=O$
Now, in order to complete their respective octets, sulfur should have one nonbonding pair of electrons and each oxygen atom should have two nonbonding pairs of electrons. Therefore, the Lewis structure of \[S{{O}_{2}}\] molecule is
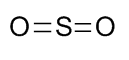
For calculating the formal charge on each atom i.e. \[S\] and \[O\], we have to use the following formula:
\[formal\;charge=valence\;electrons-non\;bonding\;electrons-\dfrac{bonding\;electrons}{2}\]
Therefore, \[formal\; charge\;on\;S=6-2-\dfrac{8}{2}=0\]
\[formal\;charge\;on\;O=6-4-\dfrac{4}{2}=0\]
Additional information: There are two types of bonds which include covalent and ionic bonds. An ionic bond is formed by the transfer of electrons i.e. one atom loses the electrons and another atom accepts the electron. For example: \[NaCl\]. On the other hand, covalent bonds are formed by sharing of electrons. Apart from these, there is one more type of bond which is called the coordinate bond in which sharing of electrons takes place between two atoms but both electrons are given by one atom.
Note:
It is important to note that the Lewis structure of \[S{{O}_{2}}\] contains four covalent bonds. In order to complete their octets, sulfur has one non-bonding pair of electrons while each oxygen has two nonbonding pairs of electrons. The formal charge on each atom i.e. sulfur and oxygen is zero.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




