
What is the final product?
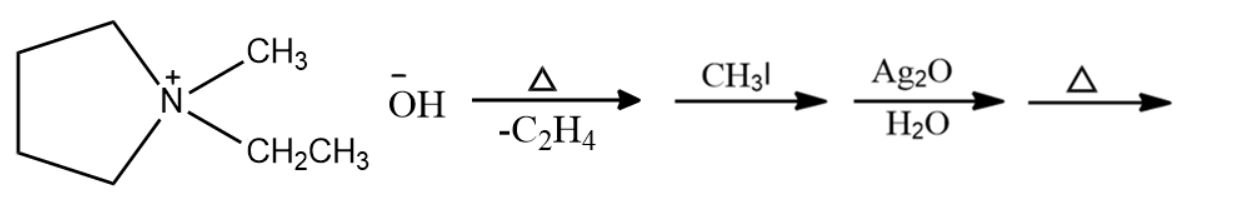

Answer
568.5k+ views
Hint: Identify the reagents used in the above series of organic reactions. Methyl Iodide is used as a methylation agent. Moist silver oxide is used to eliminate a proton and lead to the formation of a double bond. When a compound is exposed to heat, Water is eliminated thus again forming a double bond. Keep in mind the rearrangements while performing chemical reactions.
Complete Solution :
We will one reaction at a time and then finally arrive at the main product.
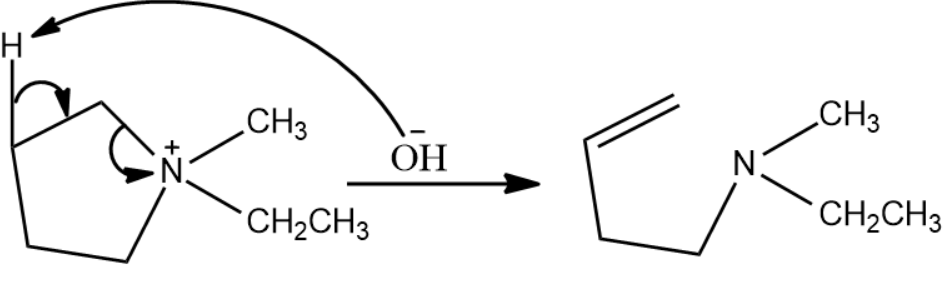
To the initial reactant, when heat is introduced, the hydroxide ion attacks replaceable hydrogen and leads to formation of an alkene and releases water as by product.
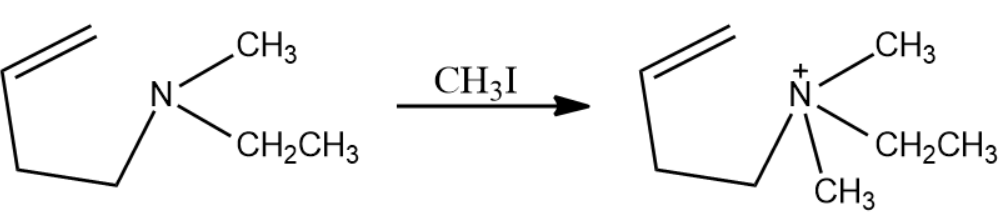
The next reagent used is methyl iodide. It is used as a methylation agent to the present lone pair on nitrogen atoms. The reaction is given below:
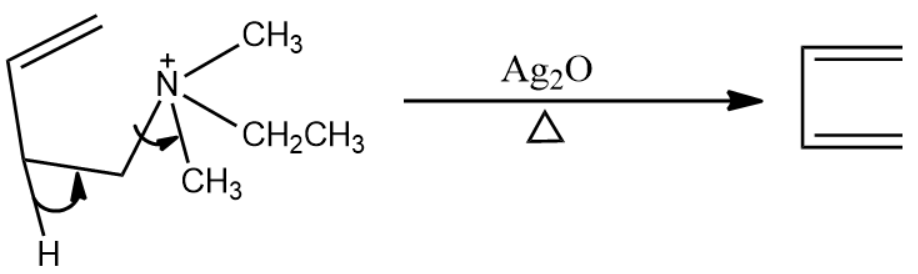
The next reagent is silver oxide in the presence of water. It is used to remove the nitrogen atom and the groups attach to it, leading to the formation of another double bond. The reaction is given below:
From the above series of reactions, we can conclude that the final product thus formed is. ${{\text{H}}_{2}}\text{C=CH}-\text{CH=C}{{\text{H}}_{2}}$
So, the correct answer is “Option A”.
Note: It is important to understand the difference between moist silver oxide and dry silver oxide when used in chemical reactions. Moist silver oxide is used for the formation of alkanol in case of alkyl halide or to remove a proton by acting as a base like the reaction given above. On the other hand, dry silver oxide is used to form ether using alkyl halides as reactants.
Complete Solution :
We will one reaction at a time and then finally arrive at the main product.
To the initial reactant, when heat is introduced, the hydroxide ion attacks replaceable hydrogen and leads to formation of an alkene and releases water as by product.

The next reagent used is methyl iodide. It is used as a methylation agent to the present lone pair on nitrogen atoms. The reaction is given below:

The next reagent is silver oxide in the presence of water. It is used to remove the nitrogen atom and the groups attach to it, leading to the formation of another double bond. The reaction is given below:

From the above series of reactions, we can conclude that the final product thus formed is. ${{\text{H}}_{2}}\text{C=CH}-\text{CH=C}{{\text{H}}_{2}}$
So, the correct answer is “Option A”.
Note: It is important to understand the difference between moist silver oxide and dry silver oxide when used in chemical reactions. Moist silver oxide is used for the formation of alkanol in case of alkyl halide or to remove a proton by acting as a base like the reaction given above. On the other hand, dry silver oxide is used to form ether using alkyl halides as reactants.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




