
What is the end product
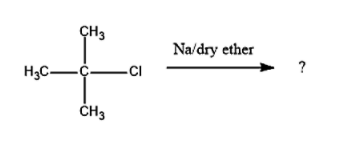

Answer
569.7k+ views
Hint: In the above given reaction, tert-butyl chloride will react with sodium in presence of dry ether, such reaction is called Wurtz reaction. Wurtz reaction is a type of coupling reaction in which the higher alkane will be formed as the product.
Complete step by step answer:
In this reaction, alkyl halide will react with sodium in the ethereal solution in order to produce the higher alkanes. This reaction is called the Wurtz reaction. Wurtz reaction is a type of coupling reaction. Wurtz reaction is used to prepare higher alkanes with an even number of carbon atoms.
The general formula for Wurtz reaction is given below:
\[2R - X + 2Na \to R - R + 2NaX\]
Consider the above reaction
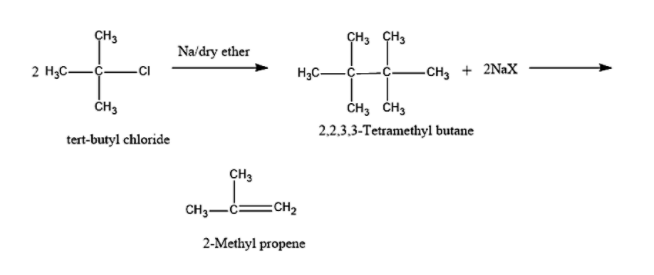
In the above reaction, two molecules of tert-butyl chloride will react with sodium and dry ether solution to form 2,2,3,3-Tetramethyl butane.
2,2,3,3-Tetramethyl butane is having three bulky methyl groups surrounding each carbon, hence there will be steric hindrance in the compound 2,2,3,3-Tetramethyl butane. In order to overcome this, the compound will undergo elimination to form 2-Methyl propene.
Additional information:
Characteristics of dry ether are given below:
- Dry ether is aprotic and polar solvent.
- Dry ether is used as a solvent for formation of Grignard reagent.
- Dry ether is used as a catalyst for Wurtz reaction.
- Dry ether is free from water.
Note: - Wurtz reaction can take place only in symmetric alkyl halide.
- If we use dissimilar alkyl halide for Wurtz reaction, then the product formed will be the mixture of alkenes.
- The separation of the above formed alkene will be difficult. It will be hard to separate these alkenes by fractional distillation because the boiling point of the product formed is very low.
Complete step by step answer:
In this reaction, alkyl halide will react with sodium in the ethereal solution in order to produce the higher alkanes. This reaction is called the Wurtz reaction. Wurtz reaction is a type of coupling reaction. Wurtz reaction is used to prepare higher alkanes with an even number of carbon atoms.
The general formula for Wurtz reaction is given below:
\[2R - X + 2Na \to R - R + 2NaX\]
Consider the above reaction

In the above reaction, two molecules of tert-butyl chloride will react with sodium and dry ether solution to form 2,2,3,3-Tetramethyl butane.
2,2,3,3-Tetramethyl butane is having three bulky methyl groups surrounding each carbon, hence there will be steric hindrance in the compound 2,2,3,3-Tetramethyl butane. In order to overcome this, the compound will undergo elimination to form 2-Methyl propene.
Additional information:
Characteristics of dry ether are given below:
- Dry ether is aprotic and polar solvent.
- Dry ether is used as a solvent for formation of Grignard reagent.
- Dry ether is used as a catalyst for Wurtz reaction.
- Dry ether is free from water.
Note: - Wurtz reaction can take place only in symmetric alkyl halide.
- If we use dissimilar alkyl halide for Wurtz reaction, then the product formed will be the mixture of alkenes.
- The separation of the above formed alkene will be difficult. It will be hard to separate these alkenes by fractional distillation because the boiling point of the product formed is very low.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




