
What is sublimation?
Answer
578.1k+ views
Hint: In sublimation the substance does not pass through the liquid state they directly move from the solid to gaseous state. Sublimation process happens when the particles of a solid absorb enough energy to overcome the forces of attraction between them.
Complete answer:
Sublimation is just another phase transition for the process changes in phases of solids, liquids, and gases. As a sublimating substance directly changes from a solid to a gas, it never passes through the liquid state. The below image represents water in its three forms: ice, water, and steam. Sublimation is therefore, just one of the ways that water or another substance can change between its potential phases.
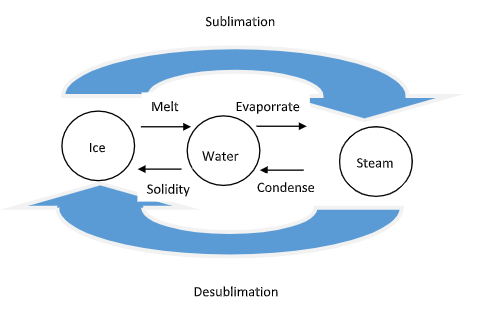
At the common and typical atmospheric pressure, we know that water is a solid at temperatures below 0 degrees Celsius, a liquid from 0 to 100 degrees Celsius, and a gas at higher temperatures. Atmospheric pressure, however, can change, particularly with altitude. Therefore, higher altitudes yield lower atmospheric pressures.
Note: Sublimation refers to the transition of a substance directly from the solid to the gaseous state., without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is referred to as deposition or desublimation, in which a substance passes directly from a gas to a solid phase.
Complete answer:
Sublimation is just another phase transition for the process changes in phases of solids, liquids, and gases. As a sublimating substance directly changes from a solid to a gas, it never passes through the liquid state. The below image represents water in its three forms: ice, water, and steam. Sublimation is therefore, just one of the ways that water or another substance can change between its potential phases.

At the common and typical atmospheric pressure, we know that water is a solid at temperatures below 0 degrees Celsius, a liquid from 0 to 100 degrees Celsius, and a gas at higher temperatures. Atmospheric pressure, however, can change, particularly with altitude. Therefore, higher altitudes yield lower atmospheric pressures.
Note: Sublimation refers to the transition of a substance directly from the solid to the gaseous state., without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is referred to as deposition or desublimation, in which a substance passes directly from a gas to a solid phase.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




