
What is Sharpless epoxidation?
Answer
489.6k+ views
Hint: Sharpless oxidation is the process of forming 2,3-epoxy alcohols from primary and secondary allylic alcohols. It is an enantioselective reaction. The stereochemistry is determined by the enantiomer of chiral tartrate diester.
Complete Step By Step Answer:
The stereochemistry of the resulting product formed can be determined by the enantiomer of the chiral tartrate diester. The tartrate ester used is diethyl tartrate (DET) or Di isopropyl tartrate. The oxidising agent used is tert-butyl hydroperoxide. The catalyst formed from titanium tetra (isopropoxide) and diethyl tartrate helps to achieve the enantioselectivity. In the presence of $3\mathop A\limits^ \circ $ molar sieves, only 5-10 mol% of the catalyst is necessary. The Sharpless epoxidation is shown by the image below:
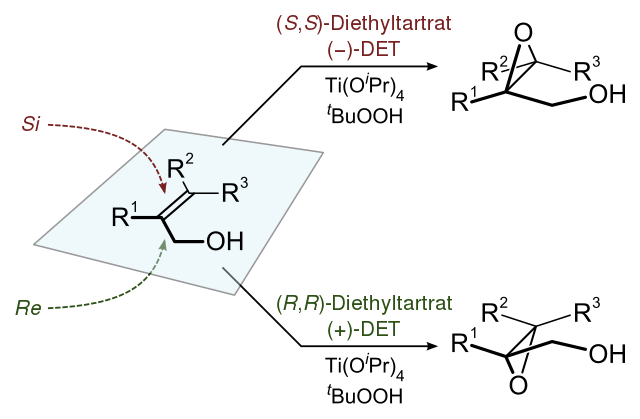
IN this process consider the allylic alcohol in the bottom right corner and other substituents in their own corners. In this orientation, the (-) diester tartrate interacts with the top half and the (+) diester tartrate interacts with the bottom half.
A successful Sharpless epoxidation can be achieved by these five major aspects. First, the formation of chiral epoxides is necessary for the synthesis of natural products, as the epoxides get easily converted into diols, ethers, etc. Second, large substrate scope. Third, the products having 90% enantiomeric excesses. Fourth, this process of Sharpless epoxidation forms predictable products. Fifth, the products formed are inexpensive and commercially available.
Note:
The structure of the catalyst used is still very uncertain. It has been concluded to be a diner of $[Ti(tartrate){(OR)_2}]$ . The structure was later found using X-ray structural determinations of model complexes that have the components to catalyse the Sharpless epoxidation.
Complete Step By Step Answer:
The stereochemistry of the resulting product formed can be determined by the enantiomer of the chiral tartrate diester. The tartrate ester used is diethyl tartrate (DET) or Di isopropyl tartrate. The oxidising agent used is tert-butyl hydroperoxide. The catalyst formed from titanium tetra (isopropoxide) and diethyl tartrate helps to achieve the enantioselectivity. In the presence of $3\mathop A\limits^ \circ $ molar sieves, only 5-10 mol% of the catalyst is necessary. The Sharpless epoxidation is shown by the image below:

IN this process consider the allylic alcohol in the bottom right corner and other substituents in their own corners. In this orientation, the (-) diester tartrate interacts with the top half and the (+) diester tartrate interacts with the bottom half.
A successful Sharpless epoxidation can be achieved by these five major aspects. First, the formation of chiral epoxides is necessary for the synthesis of natural products, as the epoxides get easily converted into diols, ethers, etc. Second, large substrate scope. Third, the products having 90% enantiomeric excesses. Fourth, this process of Sharpless epoxidation forms predictable products. Fifth, the products formed are inexpensive and commercially available.
Note:
The structure of the catalyst used is still very uncertain. It has been concluded to be a diner of $[Ti(tartrate){(OR)_2}]$ . The structure was later found using X-ray structural determinations of model complexes that have the components to catalyse the Sharpless epoxidation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




