
What is decantation?
Answer
597.3k+ views
Hint: It is a process of separation similar to sedimentation, generally carried out to separate two liquids with different densities.
Complete step by step solution:
> A process used to separate two liquids which do not mix to give a single phase or to separate a heterogeneous mixture of a solid and a liquid where the solid is sufficiently large to sediment is known as DECANTATION.
> In the case of immiscible liquids, the one having higher density settles beneath the liquid with lower density i.e. the lower density liquid is left on top and the higher density at the bottom. The lower density liquid can be poured off leaving behind the other component in the vessel.
> For example, if we take a mixture of oil and water in a beaker, a distinct layer can be observed between the two liquids after sometime as the oil layer floats on top of the water layer. The separation is carried out by pouring oil out of the container which leaves behind only the water layer.
> However, this separation process is incomplete as it is not possible to pour off all the oil without pouring some of the water therefore other alternatives such as a separatory funnel is used which has a valve at the bottom which allows draining off of the bottom layer.
In the separatory funnel, the higher density liquid moves down through the funnel into the beaker and the lower density liquid stays in the funnel once the liquids are separated, we can close the stopcock.
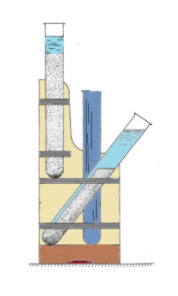
> It is also used to separate a suspension mixture by allowing gravity to pull the solid fragments to settle at the bottom. To enhance the productivity, the test tubes are placed at 45 degrees. The liquid in the tilted test tube moves upward and the residue moves towards the bottom.
Examples: Cream accelerates to the top of the milk allowing separation. Red wine is decanted from the potassium bitartrate crystals to avoid unsavoury taste.
Additional information:
A decanter centrifuge is used to separate solid-liquid continuously. As the process of decantation is time consuming, a centrifuge is used which forces the precipitate to the bottom of the container. If the force is high enough, the solid forms pellets which make the separation process easier.
NOTE: It is important to note that decantation is not sedimentation. Sedimentation refers to the process of separating solid from the liquid when the solid settles at the bottom of the container. Even though they seem quite similar, the key difference lies in the fact that sedimentation allows separation of two substances by settling whereas in decantation, two liquids could be separated due to their difference in densities.
Complete step by step solution:
> A process used to separate two liquids which do not mix to give a single phase or to separate a heterogeneous mixture of a solid and a liquid where the solid is sufficiently large to sediment is known as DECANTATION.
> In the case of immiscible liquids, the one having higher density settles beneath the liquid with lower density i.e. the lower density liquid is left on top and the higher density at the bottom. The lower density liquid can be poured off leaving behind the other component in the vessel.
> For example, if we take a mixture of oil and water in a beaker, a distinct layer can be observed between the two liquids after sometime as the oil layer floats on top of the water layer. The separation is carried out by pouring oil out of the container which leaves behind only the water layer.
> However, this separation process is incomplete as it is not possible to pour off all the oil without pouring some of the water therefore other alternatives such as a separatory funnel is used which has a valve at the bottom which allows draining off of the bottom layer.
In the separatory funnel, the higher density liquid moves down through the funnel into the beaker and the lower density liquid stays in the funnel once the liquids are separated, we can close the stopcock.
> It is also used to separate a suspension mixture by allowing gravity to pull the solid fragments to settle at the bottom. To enhance the productivity, the test tubes are placed at 45 degrees. The liquid in the tilted test tube moves upward and the residue moves towards the bottom.

Examples: Cream accelerates to the top of the milk allowing separation. Red wine is decanted from the potassium bitartrate crystals to avoid unsavoury taste.
Additional information:
A decanter centrifuge is used to separate solid-liquid continuously. As the process of decantation is time consuming, a centrifuge is used which forces the precipitate to the bottom of the container. If the force is high enough, the solid forms pellets which make the separation process easier.
NOTE: It is important to note that decantation is not sedimentation. Sedimentation refers to the process of separating solid from the liquid when the solid settles at the bottom of the container. Even though they seem quite similar, the key difference lies in the fact that sedimentation allows separation of two substances by settling whereas in decantation, two liquids could be separated due to their difference in densities.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




