
What are examples of phenols?
Answer
548.1k+ views
Hint: Phenols are defined as a compound that consists of an aromatic ring attached to a hydroxyl group. Phenols are slightly acidic than alcohol in water. It reacts with sodium hydroxide to form salts. The chemical formula of phenol is ${{C}_{6}}{{H}_{5}}OH$ .
Complete step-by-step answer:Phenols are classified into three types depending on the number of carbon-
-Monohydric phenol: this type of phenols contain only one hydroxyl group. For example: phenol
-Dihydric phenol: this type of phenols contain two hydroxyl groups. For example: Benzene$-1,2-$ diol.
-Trihydric phenol: this type of phenol contains three hydroxyl groups. For example: Benzene$-1,3,5-$ triol.
Let us see some examples of phenols-
-Picric acid- it is a compound which has a chemical formula ${{\left( N{{O}_{2}} \right)}_{3}}{{C}_{6}}{{H}_{2}}OH$ . It is the acidic phenol which has IUPAC name $2,4,6-$ trinitrophenol. Let us see the structure-
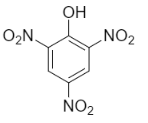
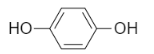
-Hydroquinone- it is an organic compound which consists of an aromatic ring attached to two hydroxyl groups at one and four positions. Its IUPAC name is benzene $-1,4-$ diol. Let us see the structures-
Note: Let us discuss the application of phenols-
-Phenols are used as an antiseptic and disinfectant.
-It is used as a precursor for synthesis of food.
-In many biological systems, phenolics are present as a component.
-Phenols are present in drugs.
-Phenols are used for the production of nylon.
A phenol shows excellent affinity towards electrophilic aromatic substitution reaction. They are very reactive because of the presence of hydroxyl groups attached to aromatic rings. Phenols are prepared from sulphuric acids and diazonium salts.
Complete step-by-step answer:Phenols are classified into three types depending on the number of carbon-
-Monohydric phenol: this type of phenols contain only one hydroxyl group. For example: phenol
-Dihydric phenol: this type of phenols contain two hydroxyl groups. For example: Benzene$-1,2-$ diol.
-Trihydric phenol: this type of phenol contains three hydroxyl groups. For example: Benzene$-1,3,5-$ triol.
Let us see some examples of phenols-
-Picric acid- it is a compound which has a chemical formula ${{\left( N{{O}_{2}} \right)}_{3}}{{C}_{6}}{{H}_{2}}OH$ . It is the acidic phenol which has IUPAC name $2,4,6-$ trinitrophenol. Let us see the structure-

-Hydroquinone- it is an organic compound which consists of an aromatic ring attached to two hydroxyl groups at one and four positions. Its IUPAC name is benzene $-1,4-$ diol. Let us see the structures-

Note: Let us discuss the application of phenols-
-Phenols are used as an antiseptic and disinfectant.
-It is used as a precursor for synthesis of food.
-In many biological systems, phenolics are present as a component.
-Phenols are present in drugs.
-Phenols are used for the production of nylon.
A phenol shows excellent affinity towards electrophilic aromatic substitution reaction. They are very reactive because of the presence of hydroxyl groups attached to aromatic rings. Phenols are prepared from sulphuric acids and diazonium salts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




