
Vitamin C is an organic acid known as:
A.Ascorbic acid
B.Citric acid
C.Glycolic acid
D.Acetic acid
Answer
576k+ views
Hint: We can use organic acid as an organic compound that has acidic properties. Some of the common organic acids are carboxylic acids, sulfonic acids. Organic compounds in biological systems showing the presence of organic groups are known as organic acids. Lactic acid, oxalic acid, formic acid, uric acid, malic acid, acetic acid are some of the common examples of organic acids.
Complete answer:
We have to know that the chemical formula of ascorbic acid is ${C_6}{H_8}{O_6}$. It is also called hexuronic acid. It appears as a solid but appears yellow in impure samples. Mild acidic conditions can be obtained when it is dissolved in water and acts as mild reducing agents. We can use ascorbic acid as an additive in foods, dietary supplements. We can create volatile compounds when we mix glucose with amino acids. The other name of ascorbic acid is Vitamin C.
Therefore, option (A) is correct.
We can draw the structure of this compound as,
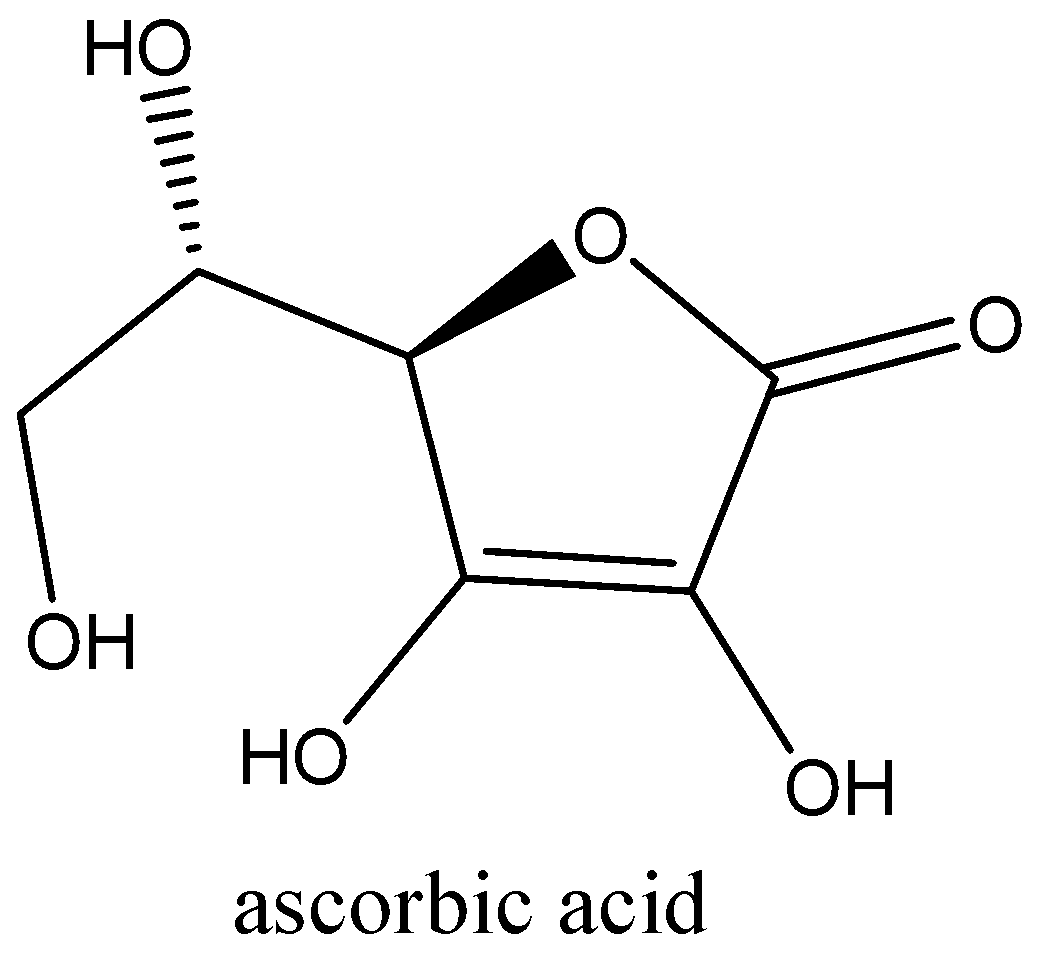
As we know that the citric acid occurs in citrus fruits. Citric acid is the reason behind the sour taste of lemons, and to lesser extent, other citrus fruits. Chemically, the difference between ascorbic acid and citric acid is that one additional oxygen atom is present in citric acid. We can use ascorbic acid and citric acid is used as food additives because they are very easy to manufacture synthetically. We use citric acid to make food tarts.
Therefore, the option (B) is incorrect.
We can draw the structure of this compound as,
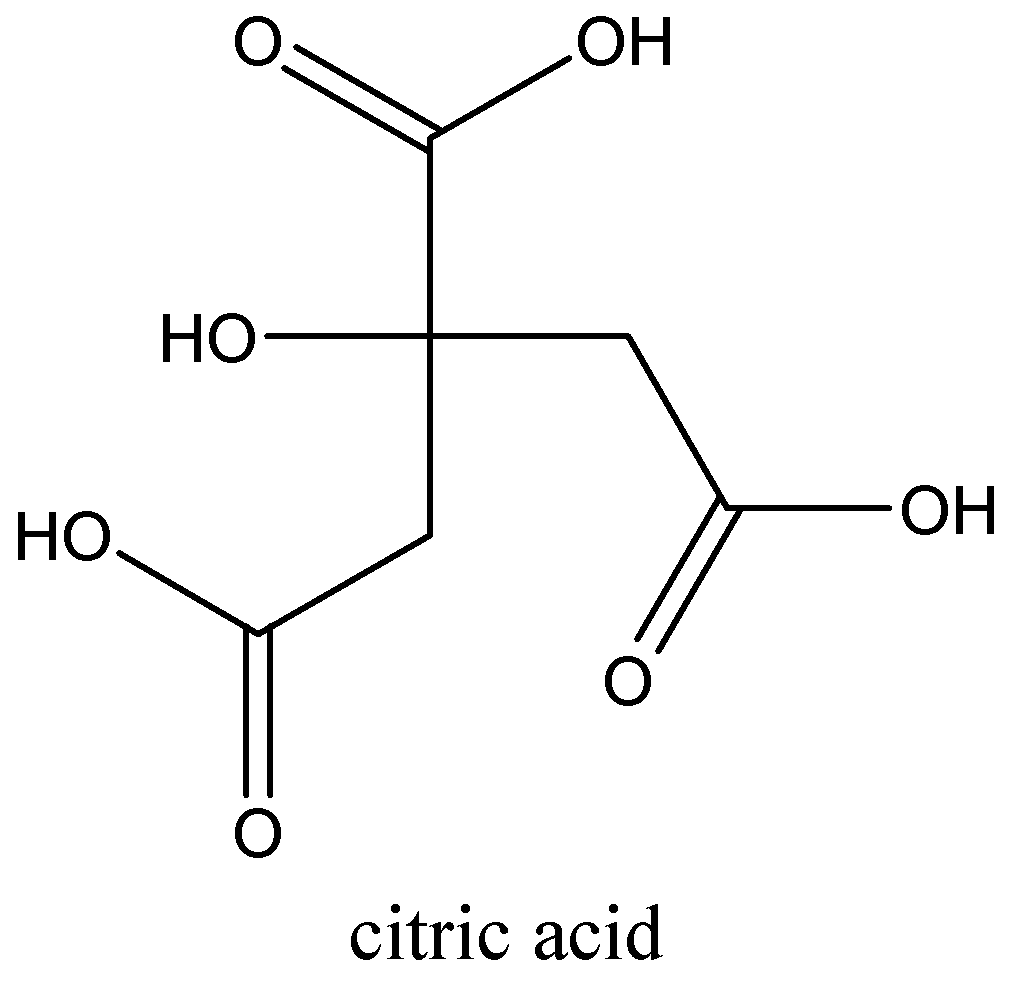
We have to know that the chemical formula of glycolic acid is ${C_2}{H_4}{O_3}$. Glycolic acid is the smallest alpha-hydroxy acid $\left( {AHA} \right)$. Glycolic acid is colourless, odorless and crystalline is highly soluble in water. It is used in skin-care products. Salt (or) ester of glycolic acid is glycolate (or) glycollate.
Therefore, the option (C) is incorrect.
We can draw the structure of this compound as,
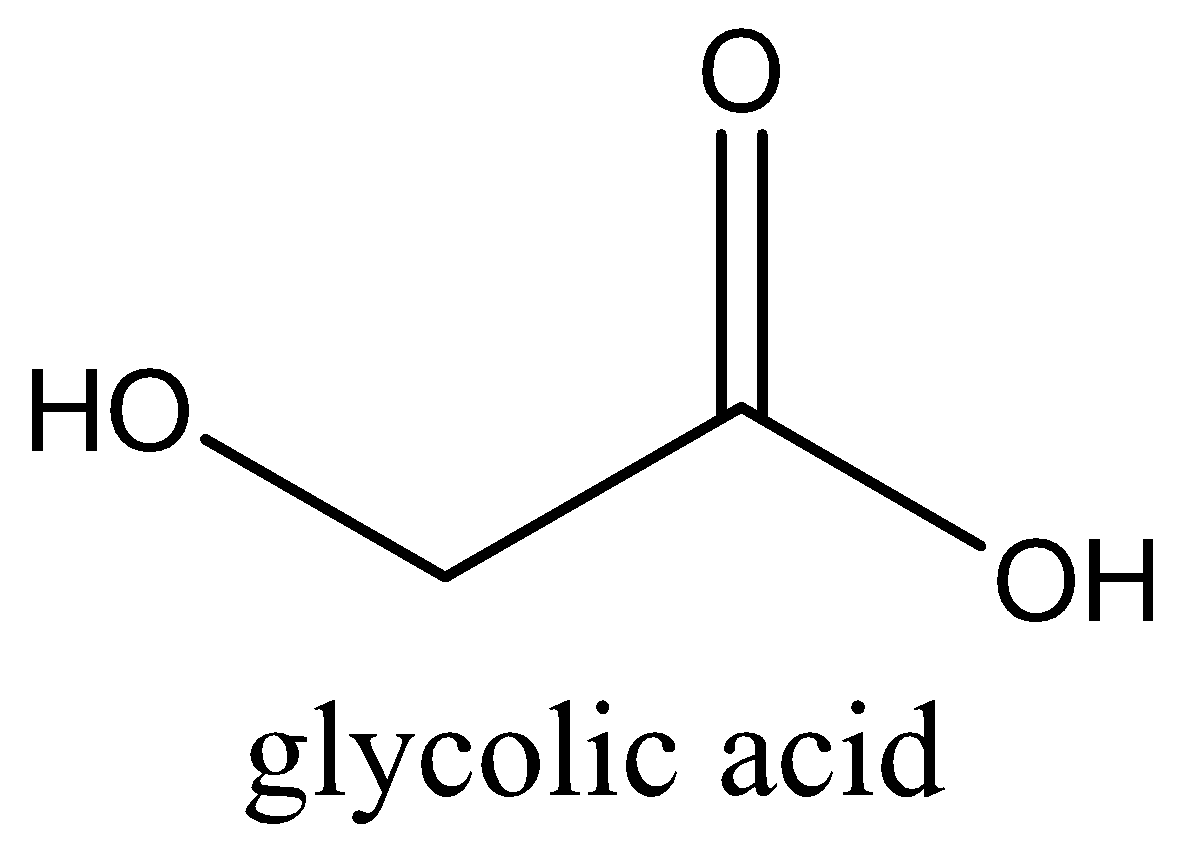
We have to know that the chemical formula of acetic acid is $C{H_3}COOH$. It appears as a colourless liquid and IUPAC name of acetic acid is ethanoic acid. Ethanoic acid is the second simplest carboxylic acid. In the food industry, we can control acetic acid by food additives.
Therefore, the option (D) is incorrect.
We can draw the structure of this compound as,
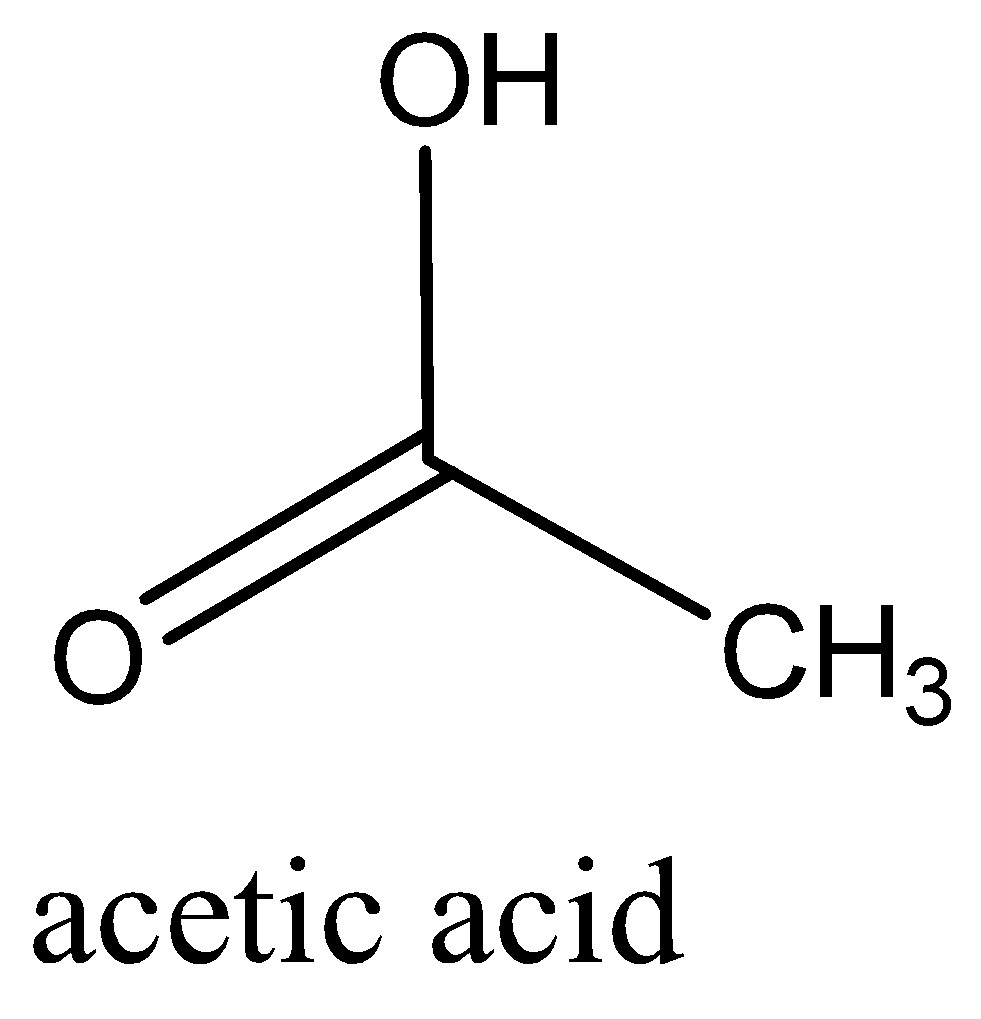
So, the correct answer is Option A.
Note: Some of the salts of ascorbic acid are sodium ascorbate, potassium ascorbate, calcium ascorbate. 1,3-diketone is formed by the nucleophilic attack of ascorbic acid on proton. Ascorbic acid is a cofactor in tyrosine oxidation. In several plants and animals, natural biosynthesis of vitamin C takes place.
Complete answer:
We have to know that the chemical formula of ascorbic acid is ${C_6}{H_8}{O_6}$. It is also called hexuronic acid. It appears as a solid but appears yellow in impure samples. Mild acidic conditions can be obtained when it is dissolved in water and acts as mild reducing agents. We can use ascorbic acid as an additive in foods, dietary supplements. We can create volatile compounds when we mix glucose with amino acids. The other name of ascorbic acid is Vitamin C.
Therefore, option (A) is correct.
We can draw the structure of this compound as,

As we know that the citric acid occurs in citrus fruits. Citric acid is the reason behind the sour taste of lemons, and to lesser extent, other citrus fruits. Chemically, the difference between ascorbic acid and citric acid is that one additional oxygen atom is present in citric acid. We can use ascorbic acid and citric acid is used as food additives because they are very easy to manufacture synthetically. We use citric acid to make food tarts.
Therefore, the option (B) is incorrect.
We can draw the structure of this compound as,

We have to know that the chemical formula of glycolic acid is ${C_2}{H_4}{O_3}$. Glycolic acid is the smallest alpha-hydroxy acid $\left( {AHA} \right)$. Glycolic acid is colourless, odorless and crystalline is highly soluble in water. It is used in skin-care products. Salt (or) ester of glycolic acid is glycolate (or) glycollate.
Therefore, the option (C) is incorrect.
We can draw the structure of this compound as,

We have to know that the chemical formula of acetic acid is $C{H_3}COOH$. It appears as a colourless liquid and IUPAC name of acetic acid is ethanoic acid. Ethanoic acid is the second simplest carboxylic acid. In the food industry, we can control acetic acid by food additives.
Therefore, the option (D) is incorrect.
We can draw the structure of this compound as,

So, the correct answer is Option A.
Note: Some of the salts of ascorbic acid are sodium ascorbate, potassium ascorbate, calcium ascorbate. 1,3-diketone is formed by the nucleophilic attack of ascorbic acid on proton. Ascorbic acid is a cofactor in tyrosine oxidation. In several plants and animals, natural biosynthesis of vitamin C takes place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




