
When vanaspati oil reacts with hydrogen then it gets converted into vanaspati ghee. In this process catalyst used is:
A.Fe
B.Mo
C.V
D.Ni
Answer
577.8k+ views
Hint: Conversion of vanaspati oil to vanaspati ghee is known as Hydrogenation. This process takes place through a metal catalyst.
Complete step by step answer:
Vegetable oils also known as vanaspati oil can undergo addition reactions due to the presence of long chains and double bonds in them. They are unsaturated compounds. Thus, heating of vegetable oils in the presence of nickel catalyst converts the unsaturated fat to saturated fats called vegetable ghee also known as vanaspati ghee. This process is called the hydrogenation of vegetable oils.
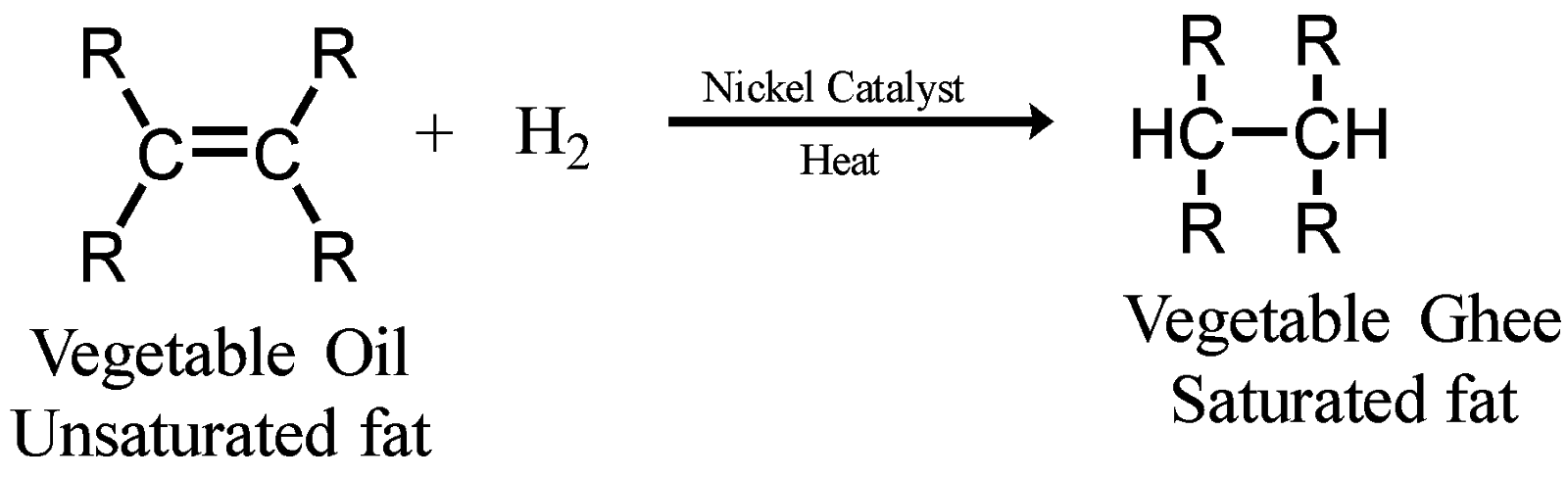
The vegetable oil is a viscous liquid whereas the vegetable ghee is semi solid/solid in nature. Here, hydrogens are added across the double bond hence this reaction is also known as addition reaction. The use of catalysts such as Nickel are used for quick and easy absorption since this metal has the capability to absorb hydrogen. The mechanism involved in this process is simple absorption of atomic hydrogen and unsaturated fat on the metal catalyst. It must be noted that hydrogen gas must be applied under pressure.
The process of hydrogenation increases the melting point of the oil or fat. Besides, resistance to oxidation and flavour deterioration is also maintained.
So, the correct option is D.
Note:
The hydrogenation of vegetable oils can also be done by platinum (Pt) catalyst in addition to Ni catalyst. Platinum catalysts can also absorb hydrogen with great ease making the hydrogenation process quick.
Complete step by step answer:
Vegetable oils also known as vanaspati oil can undergo addition reactions due to the presence of long chains and double bonds in them. They are unsaturated compounds. Thus, heating of vegetable oils in the presence of nickel catalyst converts the unsaturated fat to saturated fats called vegetable ghee also known as vanaspati ghee. This process is called the hydrogenation of vegetable oils.

The vegetable oil is a viscous liquid whereas the vegetable ghee is semi solid/solid in nature. Here, hydrogens are added across the double bond hence this reaction is also known as addition reaction. The use of catalysts such as Nickel are used for quick and easy absorption since this metal has the capability to absorb hydrogen. The mechanism involved in this process is simple absorption of atomic hydrogen and unsaturated fat on the metal catalyst. It must be noted that hydrogen gas must be applied under pressure.
The process of hydrogenation increases the melting point of the oil or fat. Besides, resistance to oxidation and flavour deterioration is also maintained.
So, the correct option is D.
Note:
The hydrogenation of vegetable oils can also be done by platinum (Pt) catalyst in addition to Ni catalyst. Platinum catalysts can also absorb hydrogen with great ease making the hydrogenation process quick.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




