
Valency of $Cr$ in $CrP{O_4}$ is:
(A). \[4\]
(B) $3$
(C) $2$
(D) $1$
Answer
586.2k+ views
Hint:Valency is the measure of the combining capacity of atoms on molecules. Therefore, it is the capacity of an atom of a single element to react and combine with a particular number of atoms of another element.
Complete step by step answer:
We know that, $CrP{O_4} \to C{r^{3 + }} + PO_4^{3 - }$
$PO_4^{3 - }$ ion is having the valency of $3$.
$x - 3 = 0$
$x = 3$
Is the valency of $Cr$ in $CrP{O_4}$.
Hence, $3$ is the answer.
Additional information:
Phosphate $\left( {{3^ - }} \right)$ is an ion that is the conjugate base of hydrogen phosphate.
It is phosphate ion and a trivalent inorganic anion. It is the conjugate base of hydrogen phosphate.
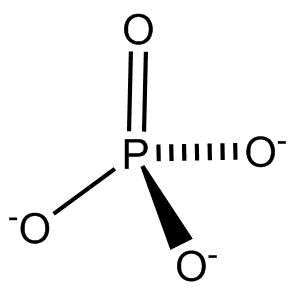
$PO_4^{3 - }$ can contribute buffering strength, sequestering power, dispersion and absorptive capabilities and solubilities.
Chromium phosphate is also used to catalyze cation exchange in sorption reaction. This catalysis is widely used in the reduction of metal toxicity during environmental clean ups. This had been applied in decreasing the concentration of lead in aquatic habitat and drinking water.
Chromium is a member of group $6$ of transition metals. The $ + 3$ and $ + 6$ occur the most commonly within chromium compounds.
Note:
High phosphate levels in the human body could lead to kidney disease, intestinal inflammation, decreased bone density, heart conditions and even premature death.
Complete step by step answer:
We know that, $CrP{O_4} \to C{r^{3 + }} + PO_4^{3 - }$
$PO_4^{3 - }$ ion is having the valency of $3$.
$x - 3 = 0$
$x = 3$
Is the valency of $Cr$ in $CrP{O_4}$.
Hence, $3$ is the answer.
Additional information:
Phosphate $\left( {{3^ - }} \right)$ is an ion that is the conjugate base of hydrogen phosphate.
It is phosphate ion and a trivalent inorganic anion. It is the conjugate base of hydrogen phosphate.

$PO_4^{3 - }$ can contribute buffering strength, sequestering power, dispersion and absorptive capabilities and solubilities.
Chromium phosphate is also used to catalyze cation exchange in sorption reaction. This catalysis is widely used in the reduction of metal toxicity during environmental clean ups. This had been applied in decreasing the concentration of lead in aquatic habitat and drinking water.
Chromium is a member of group $6$ of transition metals. The $ + 3$ and $ + 6$ occur the most commonly within chromium compounds.
Note:
High phosphate levels in the human body could lead to kidney disease, intestinal inflammation, decreased bone density, heart conditions and even premature death.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




