
Using the bond enthalpy data given below, calculate the enthalpy change for the reaction (only magnitude in nearest integer in kJ/mol),
\[{C_2}{H_4}\left( g \right) + {H_2}\left( g \right) \to {C_2}{H_6}\left( g \right)\]
Bond $C - C$ $C = C$ $C - H$ $H - H$ Bond energy \[336.81kJ/mol\] \[606.68kJ/mol\] \[410.87kJ/mol\] \[431.79kJ/mol\]
| Bond | $C - C$ | $C = C$ | $C - H$ | $H - H$ |
| Bond energy | \[336.81kJ/mol\] | \[606.68kJ/mol\] | \[410.87kJ/mol\] | \[431.79kJ/mol\] |
Answer
558.9k+ views
Hint: We need to recall the concept of bond enthalpy and understand the bond formation of the given reaction. Bond enthalpy is that which gives the energy to the chemical bond. It is the energy required to break one mole of a chemical bond. Let us consider the bond enthalpy of a single bond between hydrogen and oxygen. The total energy required to break one mole of this bond is $463kJ$. We will now study the enthalpy change in the given reaction.
Complete step by step answer:
As we know that when a bond is broken, energy is required and when a bond is created, energy is released. Hence bond breaking is considered to be endothermic and bond creating is considered to be exothermic.
The given reaction is \[{C_2}{H_4}\left( g \right) + {H_2}\left( g \right) \to {C_2}{H_6}\left( g \right)\] . We can write the skeletal structure of this reaction to understand the breaking and formation of bonds.
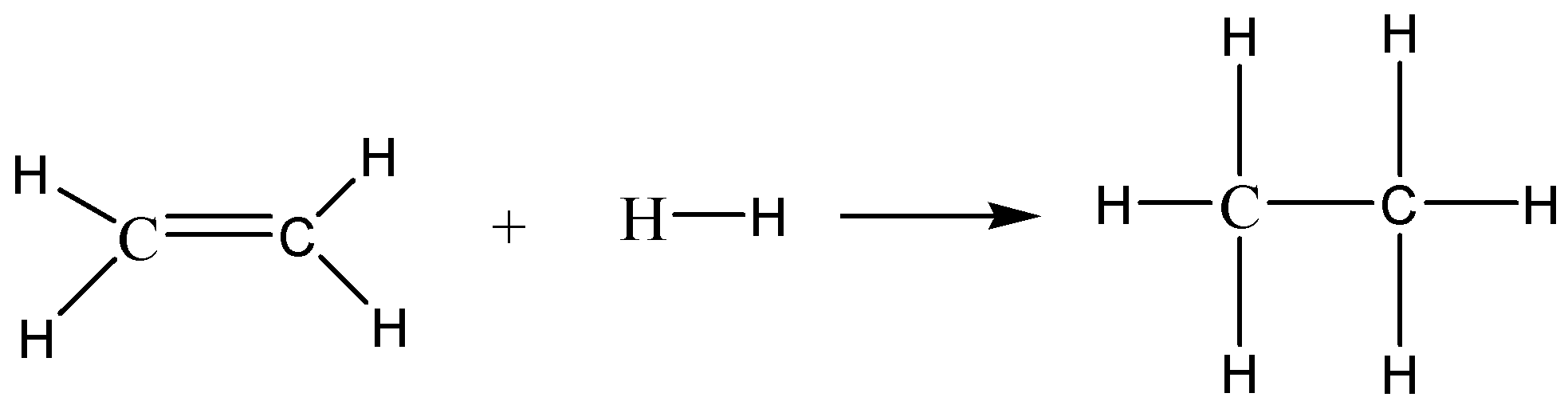
In the reactants: $1C = C$ bond, $4C - H$ bonds and $1H - H$ bond are present.
In the products: $1C - C$ bond, $6C - H$ bonds are present.
With the given values of the bond enthalpies of each bond, we can calculate the change is bond enthalpy as follows:
For the reactants: \[H = 606.68 + (4 \times 410.87) + 431.79\]= $2681.95kJ/mol$
For the products: \[H = (336.81 + 6 \times 410.87)\]= $2802.03kJ/mol$
Therefore $\Delta H = 2681.95kJ/mol - 2802.03kJ/mol$= $ - 120.08kJ/mol$
Hence the enthalpy change for the reaction of reduction of ethene to ethane is $120.08kJ/mol$(magnitude only).
Note:
It must be noted that the breaking of a chemical bond is always an endothermic process as energy is supplied to break the chemical bonds. Thus, the enthalpy change associated with the breaking of a chemical bond is always positive $\left( {\Delta H > 0} \right)$. On the other hand, the formation of a chemical bond is almost always an exothermic process. In such cases, the enthalpy change will have a negative value $\left( {\Delta H < 0} \right)$ . Hence we can say that the conversion of ethene to ethane by reduction is an exothermic process since the enthalpy change is $ - 120.08kJ/mol$ .
Complete step by step answer:
As we know that when a bond is broken, energy is required and when a bond is created, energy is released. Hence bond breaking is considered to be endothermic and bond creating is considered to be exothermic.
The given reaction is \[{C_2}{H_4}\left( g \right) + {H_2}\left( g \right) \to {C_2}{H_6}\left( g \right)\] . We can write the skeletal structure of this reaction to understand the breaking and formation of bonds.
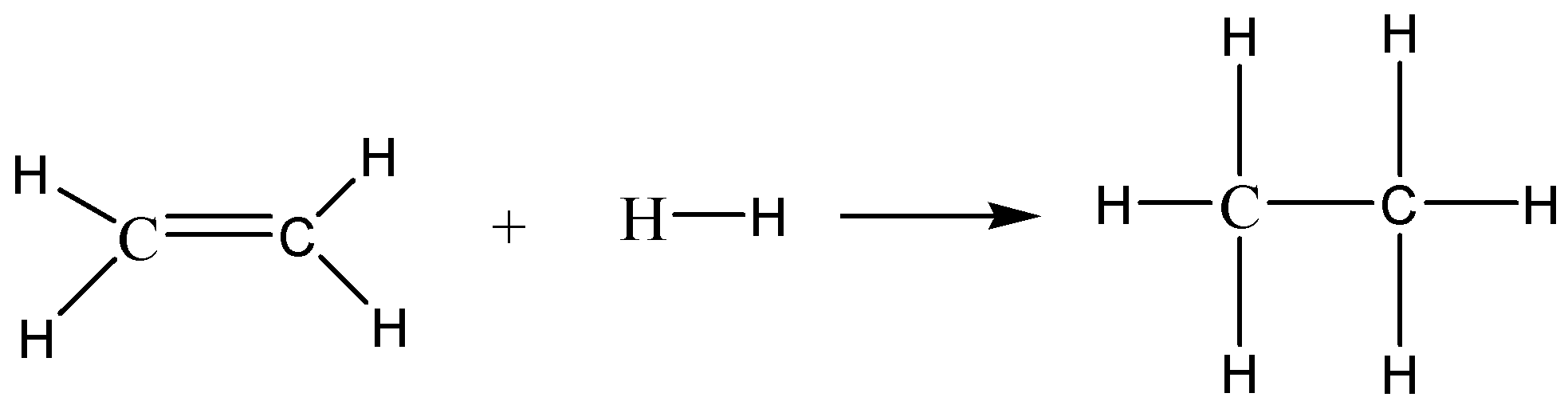
In the reactants: $1C = C$ bond, $4C - H$ bonds and $1H - H$ bond are present.
In the products: $1C - C$ bond, $6C - H$ bonds are present.
With the given values of the bond enthalpies of each bond, we can calculate the change is bond enthalpy as follows:
For the reactants: \[H = 606.68 + (4 \times 410.87) + 431.79\]= $2681.95kJ/mol$
For the products: \[H = (336.81 + 6 \times 410.87)\]= $2802.03kJ/mol$
Therefore $\Delta H = 2681.95kJ/mol - 2802.03kJ/mol$= $ - 120.08kJ/mol$
Hence the enthalpy change for the reaction of reduction of ethene to ethane is $120.08kJ/mol$(magnitude only).
Note:
It must be noted that the breaking of a chemical bond is always an endothermic process as energy is supplied to break the chemical bonds. Thus, the enthalpy change associated with the breaking of a chemical bond is always positive $\left( {\Delta H > 0} \right)$. On the other hand, the formation of a chemical bond is almost always an exothermic process. In such cases, the enthalpy change will have a negative value $\left( {\Delta H < 0} \right)$ . Hence we can say that the conversion of ethene to ethane by reduction is an exothermic process since the enthalpy change is $ - 120.08kJ/mol$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




