
Using only a periodic table as a guide, how do you write the condensed electron configuration for the following atoms?
\[A. \,Rh\]
\[B. \,Si\]
\[C. \,Hg\]
\[D. \,Hf\]
Answer
559.2k+ views
Hint: The electronic configuration says about how the electrons are distributed in atomic orbitals. The electronic configuration is filled according to the energy they have. They follow the standard notion in which electrons containing the atomic subshells are placed in a sequence. For example, the Lithium’s electronic configuration; \[Li = {\text{ }}1{s^2}2{s^1}\].
Complete step by step answer:
The standard notation yields lengthy electronic configuration (which means the electronic configuration will be written as, ( \[Na = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^1}\] ). In those cases, condensed or an abbreviated notation is used instead of using standard electronic configuration. In the condensed electron configuration, the sequence of filled subshell corresponds to the electronic configuration of a noble gas or inert gas is replaced with the symbol of that noble gas in a square bracket.
Therefore, the condensed electron configuration of sodium will be;
\[Na = {\text{ [Ne]}}\,3{s^1}\]
The full electronic configuration of any element is written in a standard format. But when it comes to higher elements it will consist of any number of orbits, so for simplification condensed electronic configuration is noted.
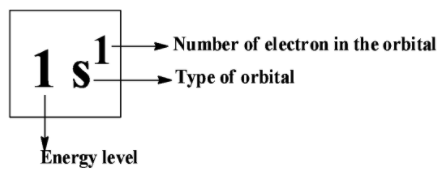
Suppose if we have considered iron as an example,
The full electronic configuration;
\[Fe = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\,3{p^6}\,4{s^2}\,3{d^6}\]
The condensed electron configuration;
\[Fe = {\text{ [Ar]}}\,{\text{4}}{s^2}3{d^6}\]
Now, you are able to differentiate that a lengthy electronic configuration can be written in abbreviated electron configuration.
So, the condensed electron configuration for the following compounds are;
\[ \Rightarrow Rh\,\, = \,\,\left[ {Kr} \right]5{s^1}4{d^8}\]
\[ \Rightarrow Si\,\, = \,\,\left[ {Ne} \right]3{s^2}3{p^2}\]
\[ \Rightarrow Hg\,\, = \,\,\left[ {Xe} \right]6{s^2}4{f^{14}}5{d^{10}}\]
\[ \Rightarrow Hf\,\, = \,\,\left[ {Xe} \right]6{s^2}4{f^{14}}5{d^2}\]
Note: The electronic configuration follows the entire principles to how to fill the electrons. The principles are Aufbau’s principle, Hund’s rule and Pauli’s exclusion principle. Each of the rule say;
1. Aufbau’s principle: In its ground state, the electrons are filled in the atomic orbitals of an atom.
2. Hund’s rule: Each orbital of the subshell should be single filled with the electron then pairing takes place. All singly occupied electrons should have the same spin.
3. Pauli’s exclusion principle: It is impossible for two electrons or more electrons to have the same values of four quantum numbers.
Complete step by step answer:
The standard notation yields lengthy electronic configuration (which means the electronic configuration will be written as, ( \[Na = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^1}\] ). In those cases, condensed or an abbreviated notation is used instead of using standard electronic configuration. In the condensed electron configuration, the sequence of filled subshell corresponds to the electronic configuration of a noble gas or inert gas is replaced with the symbol of that noble gas in a square bracket.
Therefore, the condensed electron configuration of sodium will be;
\[Na = {\text{ [Ne]}}\,3{s^1}\]
The full electronic configuration of any element is written in a standard format. But when it comes to higher elements it will consist of any number of orbits, so for simplification condensed electronic configuration is noted.

Suppose if we have considered iron as an example,
The full electronic configuration;
\[Fe = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\,3{p^6}\,4{s^2}\,3{d^6}\]
The condensed electron configuration;
\[Fe = {\text{ [Ar]}}\,{\text{4}}{s^2}3{d^6}\]
Now, you are able to differentiate that a lengthy electronic configuration can be written in abbreviated electron configuration.
So, the condensed electron configuration for the following compounds are;
\[ \Rightarrow Rh\,\, = \,\,\left[ {Kr} \right]5{s^1}4{d^8}\]
\[ \Rightarrow Si\,\, = \,\,\left[ {Ne} \right]3{s^2}3{p^2}\]
\[ \Rightarrow Hg\,\, = \,\,\left[ {Xe} \right]6{s^2}4{f^{14}}5{d^{10}}\]
\[ \Rightarrow Hf\,\, = \,\,\left[ {Xe} \right]6{s^2}4{f^{14}}5{d^2}\]
Note: The electronic configuration follows the entire principles to how to fill the electrons. The principles are Aufbau’s principle, Hund’s rule and Pauli’s exclusion principle. Each of the rule say;
1. Aufbau’s principle: In its ground state, the electrons are filled in the atomic orbitals of an atom.
2. Hund’s rule: Each orbital of the subshell should be single filled with the electron then pairing takes place. All singly occupied electrons should have the same spin.
3. Pauli’s exclusion principle: It is impossible for two electrons or more electrons to have the same values of four quantum numbers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




