
How many unpaired electrons are there in \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] \[\left( {{\text{Z = 28}}} \right)\] ?
A 0
B 8
C 2
D 4
Answer
568.8k+ views
Hint: From the atomic number of nickel, write its electronic configuration. Also write the electronic configuration of \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] cation. From the electronic configuration, determine the number of unpaired electrons.
Complete Step by step answer: The atomic number of nickel is 28. Its electronic configuration is \[\left[ {{\text{Ar}}} \right]3{d^8}4{s^2}\]
Nickel atoms lose two electrons to form \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\]cation. The electronic configuration of \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] cation is \[\left[ {{\text{Ar}}} \right]3{d^8}\] .
8 electrons are present in 3d subshell.
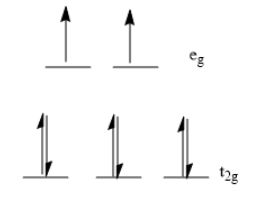
Six electrons are present in lower \[{t_{2g}}\] level and two electrons are present in upper \[{e_g}\] level.
Thus, the number of unpaired electrons in \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] ion is 2.
Hence, the correct option is the option (C).
Additional information: When 8 electrons are present in the d orbitals, 6 electrons will pair and two remain unpaired. This is irrespective of if strong field ligand is present or weak field ligand is present. Due to presence of unpaired electrons, \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] ion shows paramagnetic behaviour. If all the electrons were paired, then the ion would have been diamagnetic.
Note: The five d orbitals of a metal are degenerate. They have the same energy level. This is true in absence of ligands. In the presence of an octahedral field of ligands, the five degenerate d orbitals of metal split into two energy levels. The lower energy level contains three d orbitals and is called \[{t_{2g}}\] level. The upper energy level contains two d orbitals and is called \[{e_g}\] level.
Complete Step by step answer: The atomic number of nickel is 28. Its electronic configuration is \[\left[ {{\text{Ar}}} \right]3{d^8}4{s^2}\]
Nickel atoms lose two electrons to form \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\]cation. The electronic configuration of \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] cation is \[\left[ {{\text{Ar}}} \right]3{d^8}\] .
8 electrons are present in 3d subshell.

Six electrons are present in lower \[{t_{2g}}\] level and two electrons are present in upper \[{e_g}\] level.
Thus, the number of unpaired electrons in \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] ion is 2.
Hence, the correct option is the option (C).
Additional information: When 8 electrons are present in the d orbitals, 6 electrons will pair and two remain unpaired. This is irrespective of if strong field ligand is present or weak field ligand is present. Due to presence of unpaired electrons, \[{\text{N}}{{\text{i}}^{{\text{2 + }}}}\] ion shows paramagnetic behaviour. If all the electrons were paired, then the ion would have been diamagnetic.
Note: The five d orbitals of a metal are degenerate. They have the same energy level. This is true in absence of ligands. In the presence of an octahedral field of ligands, the five degenerate d orbitals of metal split into two energy levels. The lower energy level contains three d orbitals and is called \[{t_{2g}}\] level. The upper energy level contains two d orbitals and is called \[{e_g}\] level.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




