
What type of isomers are glucose and galactose?
Answer
526.8k+ views
Hint: As we know that isomers are molecules or polyatomic ions with same molecular formulas i.e., same number of atoms of each element but distinctive arrangements of atoms in space. One of the categories of isomers is stereoisomers which are the isomers that have the same connectivity in their atoms but a different arrangement in three-dimensional space.
Complete answer:
Let us first discuss about the isomers as follows:-
Isomers: Molecules and compounds can differ in the way the atoms of different elements are arranged as the same combination of atoms can be assembled in more than a way and these structures are known as isomers. Isomers are generally defined as the molecules with the same molecular formulas but different arrangements of atoms of various elements.
-We must know that the formula of glucose and galactose are same which is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. So both the carbohydrates are isomers of each other. Now let us draw their structures to further know that what kind of isomers they are:-
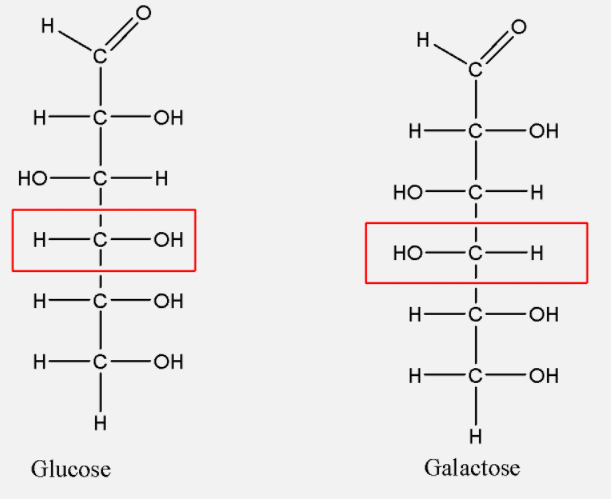
These both are stereoisomers of each other as their atoms are bonded together in the same order but they do have a different three dimensional organization of atoms around one of their asymmetric carbons (as highlighted by the red box).
Stereoisomers can be either enantiomers or diastereomers. Diastereomers are optical isomers of each other with not being mirror images of each other. Since the same can be seen in case of glucose and galactose, hence they are diastereomers.
-Therefore, glucose and galactose are diastereomers.
Note:
-Remember that enantiomers are the optically active chiral molecules which are non-superimposable mirror images of each other.
-Also fructose has the same molecular formula as glucose and galactose but they are structural isomers of each other.
Complete answer:
Let us first discuss about the isomers as follows:-
Isomers: Molecules and compounds can differ in the way the atoms of different elements are arranged as the same combination of atoms can be assembled in more than a way and these structures are known as isomers. Isomers are generally defined as the molecules with the same molecular formulas but different arrangements of atoms of various elements.
-We must know that the formula of glucose and galactose are same which is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. So both the carbohydrates are isomers of each other. Now let us draw their structures to further know that what kind of isomers they are:-

These both are stereoisomers of each other as their atoms are bonded together in the same order but they do have a different three dimensional organization of atoms around one of their asymmetric carbons (as highlighted by the red box).
Stereoisomers can be either enantiomers or diastereomers. Diastereomers are optical isomers of each other with not being mirror images of each other. Since the same can be seen in case of glucose and galactose, hence they are diastereomers.
-Therefore, glucose and galactose are diastereomers.
Note:
-Remember that enantiomers are the optically active chiral molecules which are non-superimposable mirror images of each other.
-Also fructose has the same molecular formula as glucose and galactose but they are structural isomers of each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




