
what type of bond would exist between this soap and oil?
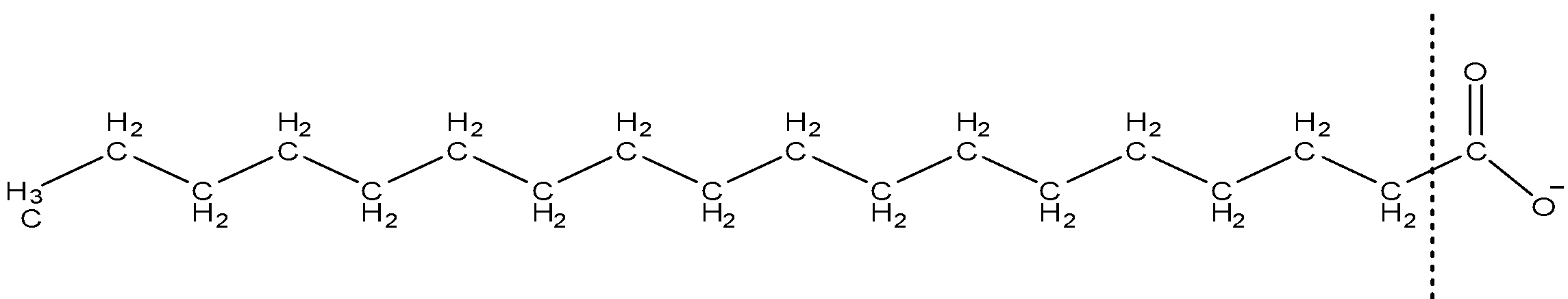
(A) Hydrogen bonds
(B) Dispersion forces
(C) Covalent bonds
(D) Dipole-dipole forces

Answer
560.7k+ views
Hint: To give the answer for the above question we first need to know the structure of soap and how they are bonded with each other. Also, there are several kinds of forces involved in different parts.
Complete step by step answer:
Soap is generally understood as sodium salts which are found from natural fatty acids. Potassium salts can also form soap but in that case, the lather will be softer. The main process of producing soap is the hydrolysis of oil.
Now the cleansing action of soap is explained according to its structure, there are two main parts of a salt molecule which is the hydrophobic (water repellent) tail and a hydrophilic (water-attracting) head. Now the tail dissolves in the oil and on the other hand, the head forms a structure similar to a ball. Now the tail removes attracts the oil forming micelles.
Now the two different parts attach with two different molecules the hydrophilic head gets attracted towards the water molecule through a strong electrostatic force of attraction. And the hydrophobic tail gets attracted towards the oil or grease through dispersion force, this is because the tail is non-polar and hence attaches to non-polar oil molecules through dispersion force while the head is polar and attaches to polar water molecules. So the type of bond which exists between the soap and oil molecule is dispersion force.
Hence, option B is correct.
Note:
Soap is always used as a cleansing agent but in hard water and soft water it acts differently in hard water the lather is not generated easily whereas in soft water the lather is formed of the generous amount.
Complete step by step answer:
Soap is generally understood as sodium salts which are found from natural fatty acids. Potassium salts can also form soap but in that case, the lather will be softer. The main process of producing soap is the hydrolysis of oil.
Now the cleansing action of soap is explained according to its structure, there are two main parts of a salt molecule which is the hydrophobic (water repellent) tail and a hydrophilic (water-attracting) head. Now the tail dissolves in the oil and on the other hand, the head forms a structure similar to a ball. Now the tail removes attracts the oil forming micelles.
Now the two different parts attach with two different molecules the hydrophilic head gets attracted towards the water molecule through a strong electrostatic force of attraction. And the hydrophobic tail gets attracted towards the oil or grease through dispersion force, this is because the tail is non-polar and hence attaches to non-polar oil molecules through dispersion force while the head is polar and attaches to polar water molecules. So the type of bond which exists between the soap and oil molecule is dispersion force.
Hence, option B is correct.
Note:
Soap is always used as a cleansing agent but in hard water and soft water it acts differently in hard water the lather is not generated easily whereas in soft water the lather is formed of the generous amount.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




