
What type of bond exists between HF, $N{{H}_{3}}$ , ${{C}_{2}}{{H}_{5}}OH$ molecules?
Answer
595.8k+ views
Hint: The difference of electronegativity between Fluorine and Hydrogen is greater than that of Hydrogen and Nitrogen or the Hydroxide group and the ethyl group. Therefore, consider the polarity of each molecule while trying to answer this question.
Complete step by step solution:
Let us look at the bond type of each of these given compounds one at a time.
- Within the HF molecule, the bond is a fairly polar covalent bond. HF is a gaseous substance made of molecules of HF and is properly described as covalent molecular on this basis. Ionic substances are at room temperature in the form of a solid, which is made of a lattice of positive and negative ions. HF is clearly not like this.
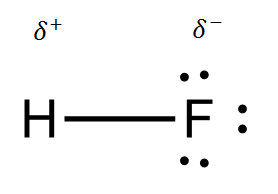
- Between the molecules of HF, there are quite strong intermolecular attractions, known as hydrogen bonds. These are a type of dipole-dipole interaction that can occur between molecules which have hydrogen bonded to one of the more electronegative elements, N, O or F. So, the partially positive hydrogen in one molecule will be attracted to the partially negative fluorine in another molecule.
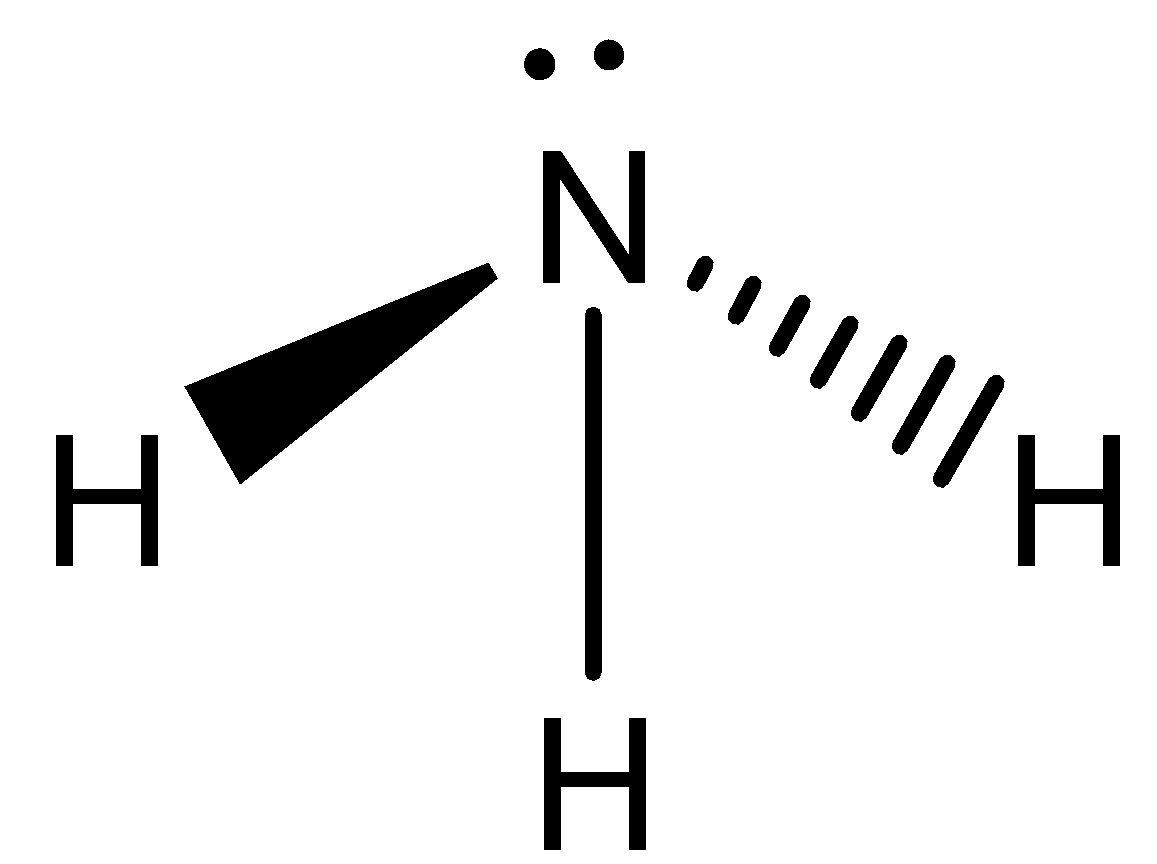
- $N{{H}_{3}}$ (Ammonia) is a covalent compound. In $N{{H}_{3}}$ , hydrogen can form 1 covalent bond while nitrogen can form 3 covalent bonds.
- As nitrogen and hydrogen both are non-metallic compounds and do not have a tendency to donate their electrons, so ‘Ammonia’ cannot be an ionic compound. Ionic compounds have a tendency to donate their electrons for the formation of ionic bonds.
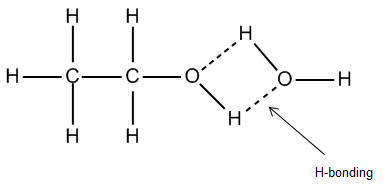
- Ethanol, or $N{{H}_{3}}$ , has two different types of bonding between its constituent atoms. The bonds between the hydrogen and carbon atoms are nonpolar covalent bonds. The hydrogen-oxygen and carbon-oxygen bonds are polar covalent bonds. In turn, these polar covalent bonds can lead to hydrogen bonds forming between ethanol molecules and some other molecules.
Note: While analysing the HF and $N{{H}_{3}}$,be very careful that these compounds cannot be ionic as Hydrogen simply does not possess enough metallic character for there to be fairly large difference in electronegativity to result in an ionic compound.
Complete step by step solution:
Let us look at the bond type of each of these given compounds one at a time.
- Within the HF molecule, the bond is a fairly polar covalent bond. HF is a gaseous substance made of molecules of HF and is properly described as covalent molecular on this basis. Ionic substances are at room temperature in the form of a solid, which is made of a lattice of positive and negative ions. HF is clearly not like this.
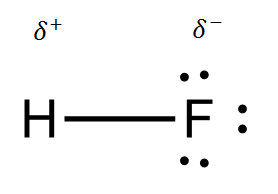
- Between the molecules of HF, there are quite strong intermolecular attractions, known as hydrogen bonds. These are a type of dipole-dipole interaction that can occur between molecules which have hydrogen bonded to one of the more electronegative elements, N, O or F. So, the partially positive hydrogen in one molecule will be attracted to the partially negative fluorine in another molecule.
- $N{{H}_{3}}$ (Ammonia) is a covalent compound. In $N{{H}_{3}}$ , hydrogen can form 1 covalent bond while nitrogen can form 3 covalent bonds.
- As nitrogen and hydrogen both are non-metallic compounds and do not have a tendency to donate their electrons, so ‘Ammonia’ cannot be an ionic compound. Ionic compounds have a tendency to donate their electrons for the formation of ionic bonds.

- Ethanol, or $N{{H}_{3}}$ , has two different types of bonding between its constituent atoms. The bonds between the hydrogen and carbon atoms are nonpolar covalent bonds. The hydrogen-oxygen and carbon-oxygen bonds are polar covalent bonds. In turn, these polar covalent bonds can lead to hydrogen bonds forming between ethanol molecules and some other molecules.

Note: While analysing the HF and $N{{H}_{3}}$,be very careful that these compounds cannot be ionic as Hydrogen simply does not possess enough metallic character for there to be fairly large difference in electronegativity to result in an ionic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




