
Two similar balls of mass ‘ $ {\text{m}} $ ’ are hung by a silk thread of length $ {\text{L}} $ and carry similar charges $ {\text{Q}} $ as in figure. Assuming the separation to be small, the separation between the balls (denoted by x) is equal to ________
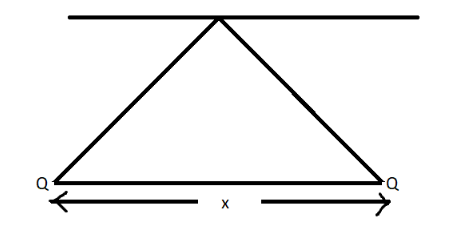

Answer
561.3k+ views
Hint: To solve this problem, we need to find the distance between both the masses. We should simply balance the forces on masses with charge $ {\text{Q}} $ .
Formula used:
We would be using the Coulomb’s law:
$ {\text{F }}\dfrac{{{\text{K}}{{\text{q}}_{\text{1}}}{{\text{q}}_{\text{2}}}}}{{{{\text{r}}^{\text{2}}}}} $
Here, $ {\text{F}} $ is the force between the masses
$ {\text{k}} $ is the Coulomb’s constant
$ {{\text{q}}_{\text{1}}}{\text{,}}{{\text{q}}_{\text{2}}} $ are the charges
$ {\text{r}} $ is the distance between the charges.
Complete step by step answer:
Let us assume that, initially the masses are hung with their respective charges and tension $ {\text{T}} $ is acting on the silk thread holding them.
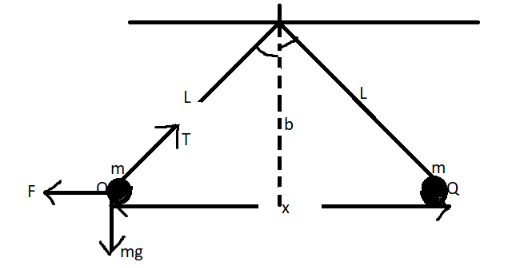
On resolving the forces in vertical and horizontal direction:
$ {\text{Tsin}}\theta {\text{ = F}} $
$ {\text{Tcos}}\theta {\text{ = mg}} $ .
By Pythagoras theorem, in the triangle above:
$ {\left( {\dfrac{{\text{x}}}{{\text{2}}}} \right)^{\text{2}}}{\text{ + }}{{\text{b}}^{\text{2}}}{\text{ = }}{{\text{L}}^{\text{2}}} $
$ \Rightarrow {\text{b = }}{\sqrt {\text{L}} ^{\text{2}}}{\text{ = L}} $
On applying Coulomb’s law,
$ {\text{F = }}\dfrac{{{\text{K}} \times {\text{Q}} \times {\text{Q}}}}{{{{\text{x}}^{\text{2}}}}} $
Now, putting these values in the resolved equations,
$ \Rightarrow \dfrac{{{\text{Tsin}}\theta }}{{{\text{Tcos}}\theta }}{\text{ = }}\dfrac{{\text{F}}}{{{\text{mg}}}} $
$ \Rightarrow {\text{mg}}\left( {\dfrac{{{\text{sin}}\theta }}{{{\text{cos}}\theta }}} \right){\text{ = }}\dfrac{{{\text{K}}{{\text{Q}}^{\text{2}}}}}{{{x^{\text{2}}}}} $
On putting the values of sine and cosine from the triangles as given in the figure:
$ \Rightarrow {\text{mg}}\left( {\dfrac{{\text{x}}}{{{\text{2L}}}}} \right){\text{ = }}\dfrac{{{\text{K}}{{\text{Q}}^{\text{2}}}}}{{{{\text{r}}^{\text{2}}}}} $
$ \Rightarrow {\text{mg}}\left( {\dfrac{{{{\text{x}}^{\text{3}}}}}{{{\text{mg}}}}} \right){\text{ = }}\dfrac{{{\text{K}}{{\text{Q}}^{\text{2}}} \times {\text{2L}}}}{{\text{1}}} $
On putting the value of, $ {\text{K = }}\dfrac{{\text{1}}}{{{\text{4}}\pi {\varepsilon _{\text{0}}}}} $
$ {\text{x = }}{\left( {\dfrac{{\text{1}}}{{{\text{4}}\pi {\varepsilon _{\text{0}}}}}\dfrac{{{{\text{Q}}^{\text{2}}}{\text{L}}}}{{{\text{mg}}}}} \right)^{{\text{1/3}}}} $
So, the distance between the masses is $ {\text{x = }}{\left( {\dfrac{{\text{1}}}{{{\text{4}}\pi {\varepsilon _{\text{0}}}}}\dfrac{{{{\text{Q}}^{\text{2}}}{\text{L}}}}{{{\text{mg}}}}} \right)^{{\text{1/3}}}} $ .
Additional Information
Coulomb's law, or Coulomb's inverse-square law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventionally called electrostatic force or Coulomb force. The law was first discovered in 1785 by French physicist Charles-Augustin de Coulomb, hence the name. Coulomb's law was essential to the development of the theory of electromagnetism
Note:
We should also note that the value of K should always be replaced to cancel other values. Also, the force is along the straight line joining the two charges. If the charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive.
Formula used:
We would be using the Coulomb’s law:
$ {\text{F }}\dfrac{{{\text{K}}{{\text{q}}_{\text{1}}}{{\text{q}}_{\text{2}}}}}{{{{\text{r}}^{\text{2}}}}} $
Here, $ {\text{F}} $ is the force between the masses
$ {\text{k}} $ is the Coulomb’s constant
$ {{\text{q}}_{\text{1}}}{\text{,}}{{\text{q}}_{\text{2}}} $ are the charges
$ {\text{r}} $ is the distance between the charges.
Complete step by step answer:
Let us assume that, initially the masses are hung with their respective charges and tension $ {\text{T}} $ is acting on the silk thread holding them.

On resolving the forces in vertical and horizontal direction:
$ {\text{Tsin}}\theta {\text{ = F}} $
$ {\text{Tcos}}\theta {\text{ = mg}} $ .
By Pythagoras theorem, in the triangle above:
$ {\left( {\dfrac{{\text{x}}}{{\text{2}}}} \right)^{\text{2}}}{\text{ + }}{{\text{b}}^{\text{2}}}{\text{ = }}{{\text{L}}^{\text{2}}} $
$ \Rightarrow {\text{b = }}{\sqrt {\text{L}} ^{\text{2}}}{\text{ = L}} $
On applying Coulomb’s law,
$ {\text{F = }}\dfrac{{{\text{K}} \times {\text{Q}} \times {\text{Q}}}}{{{{\text{x}}^{\text{2}}}}} $
Now, putting these values in the resolved equations,
$ \Rightarrow \dfrac{{{\text{Tsin}}\theta }}{{{\text{Tcos}}\theta }}{\text{ = }}\dfrac{{\text{F}}}{{{\text{mg}}}} $
$ \Rightarrow {\text{mg}}\left( {\dfrac{{{\text{sin}}\theta }}{{{\text{cos}}\theta }}} \right){\text{ = }}\dfrac{{{\text{K}}{{\text{Q}}^{\text{2}}}}}{{{x^{\text{2}}}}} $
On putting the values of sine and cosine from the triangles as given in the figure:
$ \Rightarrow {\text{mg}}\left( {\dfrac{{\text{x}}}{{{\text{2L}}}}} \right){\text{ = }}\dfrac{{{\text{K}}{{\text{Q}}^{\text{2}}}}}{{{{\text{r}}^{\text{2}}}}} $
$ \Rightarrow {\text{mg}}\left( {\dfrac{{{{\text{x}}^{\text{3}}}}}{{{\text{mg}}}}} \right){\text{ = }}\dfrac{{{\text{K}}{{\text{Q}}^{\text{2}}} \times {\text{2L}}}}{{\text{1}}} $
On putting the value of, $ {\text{K = }}\dfrac{{\text{1}}}{{{\text{4}}\pi {\varepsilon _{\text{0}}}}} $
$ {\text{x = }}{\left( {\dfrac{{\text{1}}}{{{\text{4}}\pi {\varepsilon _{\text{0}}}}}\dfrac{{{{\text{Q}}^{\text{2}}}{\text{L}}}}{{{\text{mg}}}}} \right)^{{\text{1/3}}}} $
So, the distance between the masses is $ {\text{x = }}{\left( {\dfrac{{\text{1}}}{{{\text{4}}\pi {\varepsilon _{\text{0}}}}}\dfrac{{{{\text{Q}}^{\text{2}}}{\text{L}}}}{{{\text{mg}}}}} \right)^{{\text{1/3}}}} $ .
Additional Information
Coulomb's law, or Coulomb's inverse-square law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventionally called electrostatic force or Coulomb force. The law was first discovered in 1785 by French physicist Charles-Augustin de Coulomb, hence the name. Coulomb's law was essential to the development of the theory of electromagnetism
Note:
We should also note that the value of K should always be replaced to cancel other values. Also, the force is along the straight line joining the two charges. If the charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




