
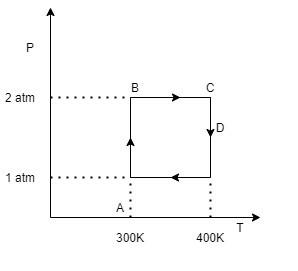
Two moles of helium gas undergo a reversible cyclic process as shown in figure assuming gas to be ideal. What is the work for the process C to D?
(A) $-800$ Rln2
(B) Zero
(C) $+200$ Rln2
(D) $-600$ Rln2

Answer
559.5k+ views
Hint: Use the formula for the work done for reversible process which is given as $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}$. Since we have to find work done from C to D, as we can see temperature remains constant for this process so we can use Boyle’s Law here for replacing the volume into pressure. Use the initial and final pressure values for the process C to D to obtain the required work done.
Formula Used: $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}=-nRT\ln \dfrac{{{P}_{i}}}{{{P}_{f}}}$, Boyle’s Law: PV = constant (at same temperature conditions)
Where n is no. of moles
R is gas constant
T is temperature in kelvin
${{V} {f}}\, and\, {{V} {i}} $are final and initial volumes respectively
${{P} {i}}\, and\, {{P} {f}} $are initial and final pressures respectively
Complete Step By Step Solution:
No. of moles of helium gas given are 2. Since we have the formula for reversible work done in the form of initial and final volumes $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}$ but according to the graph we have different values of pressure for various processes so we will rewrite the work done formula in terms of pressure. We can use Boyle’s Law here which states that pressure and volume of a gas varies inversely with each other provided temperature is constant i.e. PV = constant or in terms of initial and final values we can say that, $\begin{align}
& {{P}_{i}}{{V}_{i}}={{P}_{f}}{{V}_{f}} \\
& \dfrac{{{P}_{i}}}{{{P}_{f}}}=\dfrac{{{V}_{f}}}{{{V}_{i}}} \\
\end{align}$. So replacing volume with pressure in work done formula we get $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}=-nRT\ln \dfrac{{{P}_{i}}}{{{P}_{f}}}$. For the process from C to D, temperature remains constant i.e. equal to 400K. Initial value of pressure at C is 2atm and final value of pressure at D is 1atm. So, obtaining the work done we get $\begin {align}
& W=-nRT\ln \dfrac{{{P}_{i}}}{{{P}_{f}}} \\
& \,\,\,\,\,\,=-2R\times 400\ln \dfrac{2}{1} \\
& \,\,\,\,\,\,=-800R\ln 2 \\
& \\
\end{align}$
So, the correct choice is (A).
Note: In the formula for work done if we want to consider volume then it is ratio of final volume over initial volume but if we are considering pressure then it is ratio of initial pressure over final pressure. Reversible process is one which can return to its initial position without introducing any changes in the thermodynamic properties of the universe. Perfectly reversible processes do not exist as they take infinite amounts of time to finish.
Formula Used: $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}=-nRT\ln \dfrac{{{P}_{i}}}{{{P}_{f}}}$, Boyle’s Law: PV = constant (at same temperature conditions)
Where n is no. of moles
R is gas constant
T is temperature in kelvin
${{V} {f}}\, and\, {{V} {i}} $are final and initial volumes respectively
${{P} {i}}\, and\, {{P} {f}} $are initial and final pressures respectively
Complete Step By Step Solution:
No. of moles of helium gas given are 2. Since we have the formula for reversible work done in the form of initial and final volumes $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}$ but according to the graph we have different values of pressure for various processes so we will rewrite the work done formula in terms of pressure. We can use Boyle’s Law here which states that pressure and volume of a gas varies inversely with each other provided temperature is constant i.e. PV = constant or in terms of initial and final values we can say that, $\begin{align}
& {{P}_{i}}{{V}_{i}}={{P}_{f}}{{V}_{f}} \\
& \dfrac{{{P}_{i}}}{{{P}_{f}}}=\dfrac{{{V}_{f}}}{{{V}_{i}}} \\
\end{align}$. So replacing volume with pressure in work done formula we get $W=-nRT\ln \dfrac{{{V}_{f}}}{{{V}_{i}}}=-nRT\ln \dfrac{{{P}_{i}}}{{{P}_{f}}}$. For the process from C to D, temperature remains constant i.e. equal to 400K. Initial value of pressure at C is 2atm and final value of pressure at D is 1atm. So, obtaining the work done we get $\begin {align}
& W=-nRT\ln \dfrac{{{P}_{i}}}{{{P}_{f}}} \\
& \,\,\,\,\,\,=-2R\times 400\ln \dfrac{2}{1} \\
& \,\,\,\,\,\,=-800R\ln 2 \\
& \\
\end{align}$
So, the correct choice is (A).
Note: In the formula for work done if we want to consider volume then it is ratio of final volume over initial volume but if we are considering pressure then it is ratio of initial pressure over final pressure. Reversible process is one which can return to its initial position without introducing any changes in the thermodynamic properties of the universe. Perfectly reversible processes do not exist as they take infinite amounts of time to finish.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




