
When two ice cubes are pressed together, they join together. Identify the type of attraction which helps for the above process.
A. Vanderwaals
B. Covalent bond
C. Dative bond
D. Hydrogen bond
Answer
571.8k+ views
Hint: We know that the force of attraction that holds atoms, molecules or ions is termed as chemical bond. The four types of intermolecular forces are ionic bonds, hydrogen bonds, dipole dipole interaction and Vander Waal dispersion force.
Complete step by step answer:
Let’s discuss hydrogen bonds in detail. Hydrogen bond is a chemical bond in which formation of a covalent link of hydrogen atoms with other electronegative atoms, such as fluorine, nitrogen and oxygen atoms takes place in the same or another molecule.
Now, come to the question. The pressing of the two ice cubes results in the joining of the two ice cubes due to the formation of hydrogen bonds between water molecules. The hydrogen bonding can in water can be shown as follows:
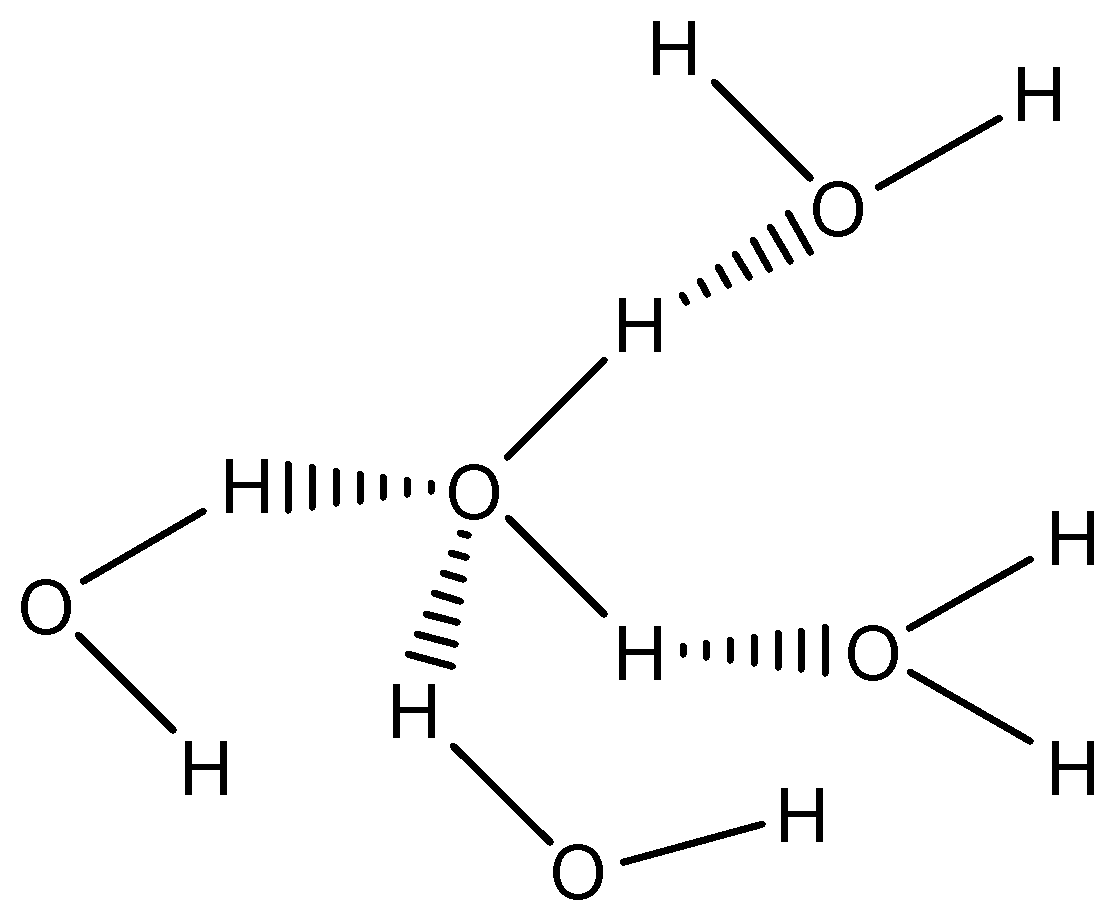
One water molecule forms hydrogen bonds with four other water molecules, that is, intermolecular H-bonding. Due to this, the two ice cubes joined.
So, the correct answer is Option D.
Additional Information:
The order of strengths of four intermolecular forces is,
Ionic bonds>Hydrogen bond> dipole dipole interaction> Vander Waal dispersion force
Ionic bonding is the strongest force of attraction as this force is due to the attraction of positive and negative ions. Van Der Waals force is the weakest force of attraction.
Note: There are two types of hydrogen bonding, namely intermolecular H-bonding and intramolecular H-bonding. If the formation of hydrogen bonding takes place within the same molecule, then the type of H-bonding is intramolecular H-bonding. If the formation of H-bonding takes place between different molecules, then the type of H-bonding is intermolecular H-bonding.
Complete step by step answer:
Let’s discuss hydrogen bonds in detail. Hydrogen bond is a chemical bond in which formation of a covalent link of hydrogen atoms with other electronegative atoms, such as fluorine, nitrogen and oxygen atoms takes place in the same or another molecule.
Now, come to the question. The pressing of the two ice cubes results in the joining of the two ice cubes due to the formation of hydrogen bonds between water molecules. The hydrogen bonding can in water can be shown as follows:

One water molecule forms hydrogen bonds with four other water molecules, that is, intermolecular H-bonding. Due to this, the two ice cubes joined.
So, the correct answer is Option D.
Additional Information:
The order of strengths of four intermolecular forces is,
Ionic bonds>Hydrogen bond> dipole dipole interaction> Vander Waal dispersion force
Ionic bonding is the strongest force of attraction as this force is due to the attraction of positive and negative ions. Van Der Waals force is the weakest force of attraction.
Note: There are two types of hydrogen bonding, namely intermolecular H-bonding and intramolecular H-bonding. If the formation of hydrogen bonding takes place within the same molecule, then the type of H-bonding is intramolecular H-bonding. If the formation of H-bonding takes place between different molecules, then the type of H-bonding is intermolecular H-bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




