
Treatment of $N{{H}_{3}}$ with 1, 4-dibromobutane yields:
(a)- ${{({{C}_{4}}{{H}_{9}})}_{4}}NBr$
(b)- ${{H}_{2}}NC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}$
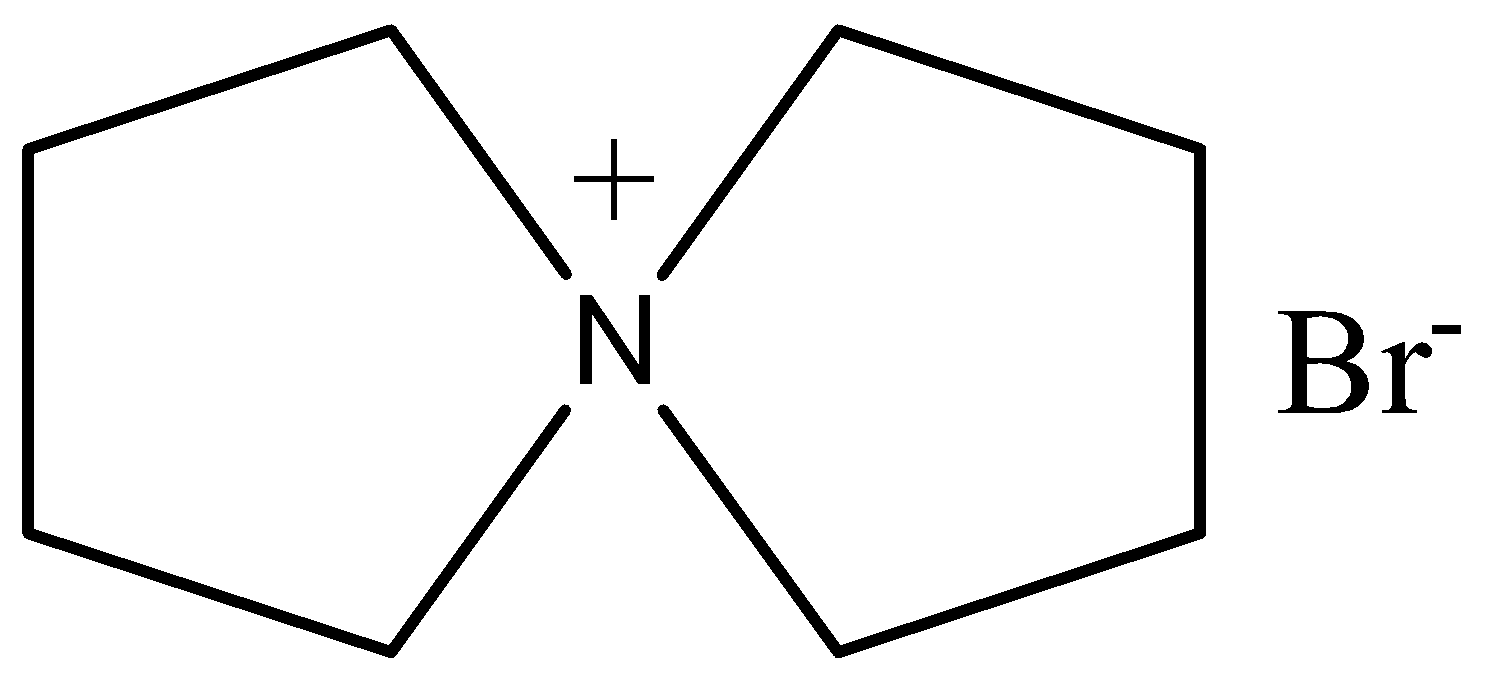
(c)-
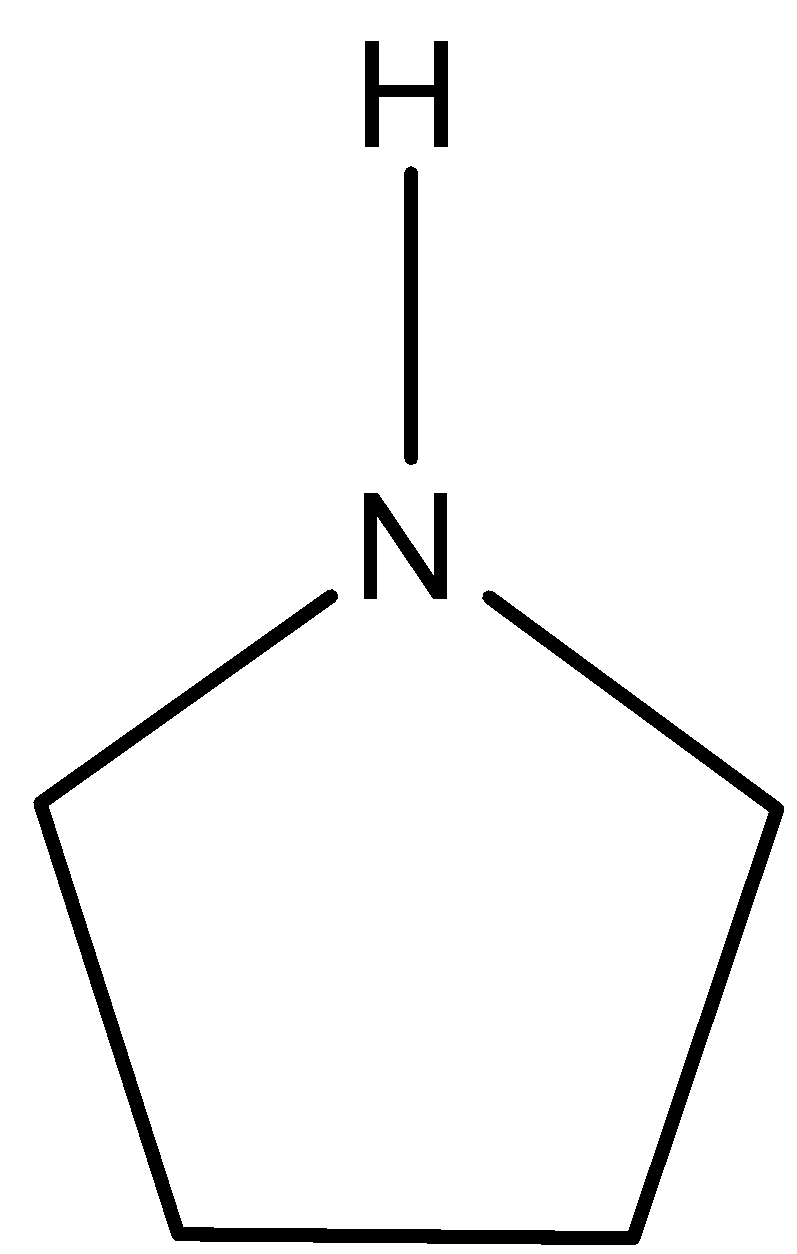
(d)-


Answer
565.5k+ views
Hint: When the amine reacts with alkyl halide there is a formation of a quaternary ammonium salt and this reaction is known as Hoffmann's ammonolysis or Hoffmann’s exhaustive alkylation reaction.
Complete answer:
When the amine reacts with alkyl halide there is a formation of a quaternary ammonium salt and this reaction is known as Hoffmann's ammonolysis or Hoffmann’s exhaustive alkylation reaction. Quaternary ammonium salts are those compounds in which the nitrogen doesn’t have any hydrogen atom and the nitrogen atom has a positive charge.
The reaction actually takes place by the formation of a series of primary amine, secondary amine, tertiary amine, and then quaternary ammonium salt. The general reaction is given below:
${{H}_{3}}N+R-X\xrightarrow{373K}R{{N}^{+}}{{H}_{3}}{{X}^{-}}RN{{H}_{2}}+NH_{4}^{+}{{X}^{-}}$
$R-N{{H}_{2}}+R-X\xrightarrow{373K}{{R}_{2}}NH_{2}^{+}{{X}^{-}}{{R}_{2}}NH+NH_{4}^{+}{{X}^{-}}$
${{R}_{2}}NH+R-X\xrightarrow{373K}{{R}_{3}}N{{H}^{+}}{{X}^{-}}{{R}_{3}}N+NH_{4}^{+}{{X}^{-}}$
${{R}_{3}}N+R-X\xrightarrow{373K}{{R}_{4}}{{N}^{+}}{{X}^{-}}$
So these are the general steps but the actual composition only depends upon the reactants taken. In this reaction, the alkyl group is replaced with the amine group.
So in 1, 4-dibromobutane there are two alkyl groups, so both the atoms are replaced and form a ring structure. In 1, 4-dibromobutane, there are four carbon atoms, so a ring structure of five atoms in which four are carbon atoms and one is nitrogen atom with a positive charge. So the reaction is given below:
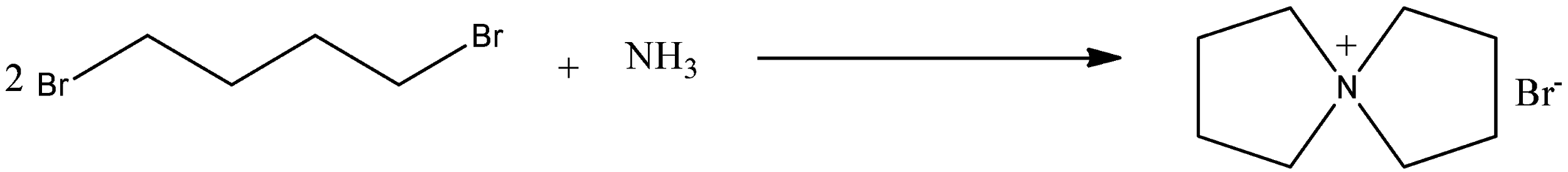
So the product is known as 5-azoniaspiro [4, 4]nonane bromide. This reaction follows through the bimolecular substitution reaction.
Therefore, the correct answer is an option (c).
Note:
In Hoffmann’s ammonolysis reaction, the aryl halides do not react because the aryl halides are less reactive than the alkyl halides. The mixtures formed in this reaction are very difficult to separate.
Complete answer:
When the amine reacts with alkyl halide there is a formation of a quaternary ammonium salt and this reaction is known as Hoffmann's ammonolysis or Hoffmann’s exhaustive alkylation reaction. Quaternary ammonium salts are those compounds in which the nitrogen doesn’t have any hydrogen atom and the nitrogen atom has a positive charge.
The reaction actually takes place by the formation of a series of primary amine, secondary amine, tertiary amine, and then quaternary ammonium salt. The general reaction is given below:
${{H}_{3}}N+R-X\xrightarrow{373K}R{{N}^{+}}{{H}_{3}}{{X}^{-}}RN{{H}_{2}}+NH_{4}^{+}{{X}^{-}}$
$R-N{{H}_{2}}+R-X\xrightarrow{373K}{{R}_{2}}NH_{2}^{+}{{X}^{-}}{{R}_{2}}NH+NH_{4}^{+}{{X}^{-}}$
${{R}_{2}}NH+R-X\xrightarrow{373K}{{R}_{3}}N{{H}^{+}}{{X}^{-}}{{R}_{3}}N+NH_{4}^{+}{{X}^{-}}$
${{R}_{3}}N+R-X\xrightarrow{373K}{{R}_{4}}{{N}^{+}}{{X}^{-}}$
So these are the general steps but the actual composition only depends upon the reactants taken. In this reaction, the alkyl group is replaced with the amine group.
So in 1, 4-dibromobutane there are two alkyl groups, so both the atoms are replaced and form a ring structure. In 1, 4-dibromobutane, there are four carbon atoms, so a ring structure of five atoms in which four are carbon atoms and one is nitrogen atom with a positive charge. So the reaction is given below:
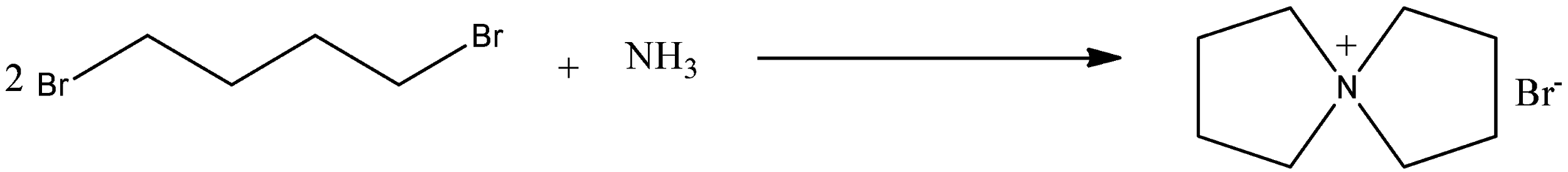
So the product is known as 5-azoniaspiro [4, 4]nonane bromide. This reaction follows through the bimolecular substitution reaction.
Therefore, the correct answer is an option (c).
Note:
In Hoffmann’s ammonolysis reaction, the aryl halides do not react because the aryl halides are less reactive than the alkyl halides. The mixtures formed in this reaction are very difficult to separate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




