
How many transition states are there in the mechanism for the acid-catalyzed hydration of an alkene?
Answer
537.3k+ views
Hint: When the alkene is hydrated or treated with water in the presence of an acid, then there is the formation of alcohol, in this reaction, there are three steps, i.e., the addition of a proton to the alkene from hydronium ion, water molecule attacks the carbocation, and removal of a hydrogen ion from the oxonium.
Complete answer:
When the alkene is hydrated or treated with water in the presence of an acid, then there is the formation of alcohol, in this reaction, there are three steps, i.e., the addition of a proton to the alkene from hydronium ion, water molecule attacks the carbocation, and removal of a hydrogen ion from the oxonium ion.
All the steps given above have a transition state.
The hydration of alkene involves strong acids like hydrochloric acid (HCl), sulfuric acid (${{H}_{2}}S{{O}_{4}}$), etc. The proton from reacts with water to form hydronium ion (${{H}_{3}}{{O}^{+}}$), as shown below:
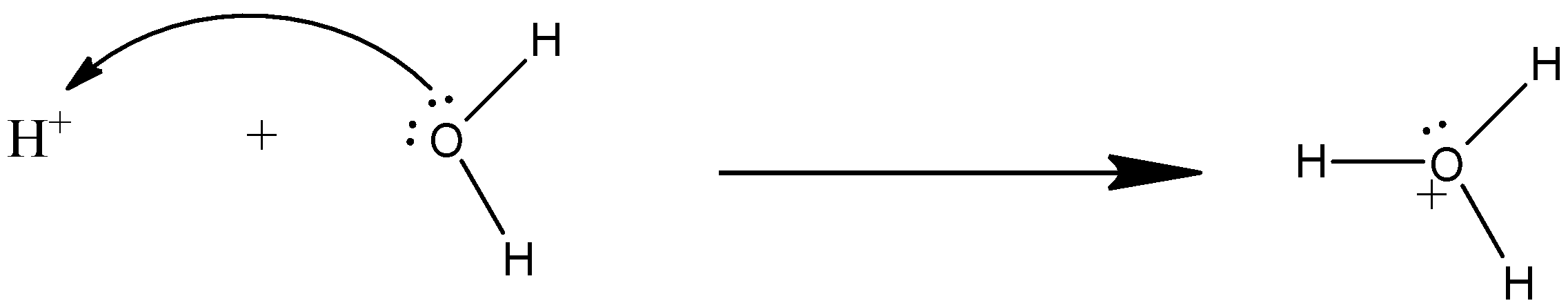
The mechanism takes place as, first, in the alkene the double breaks, and at one carbon atom there will be the addition of hydrogen ion from the hydronium ion and the other carbon atom will have a positive charge. Now, at this positive charge, the water molecule will attack. This will create a positive charge on the oxygen atom, so another water molecule will take a hydrogen ion from the oxonium ion. In all three steps transition states are formed and alcohol is formed. The reactions are given below:
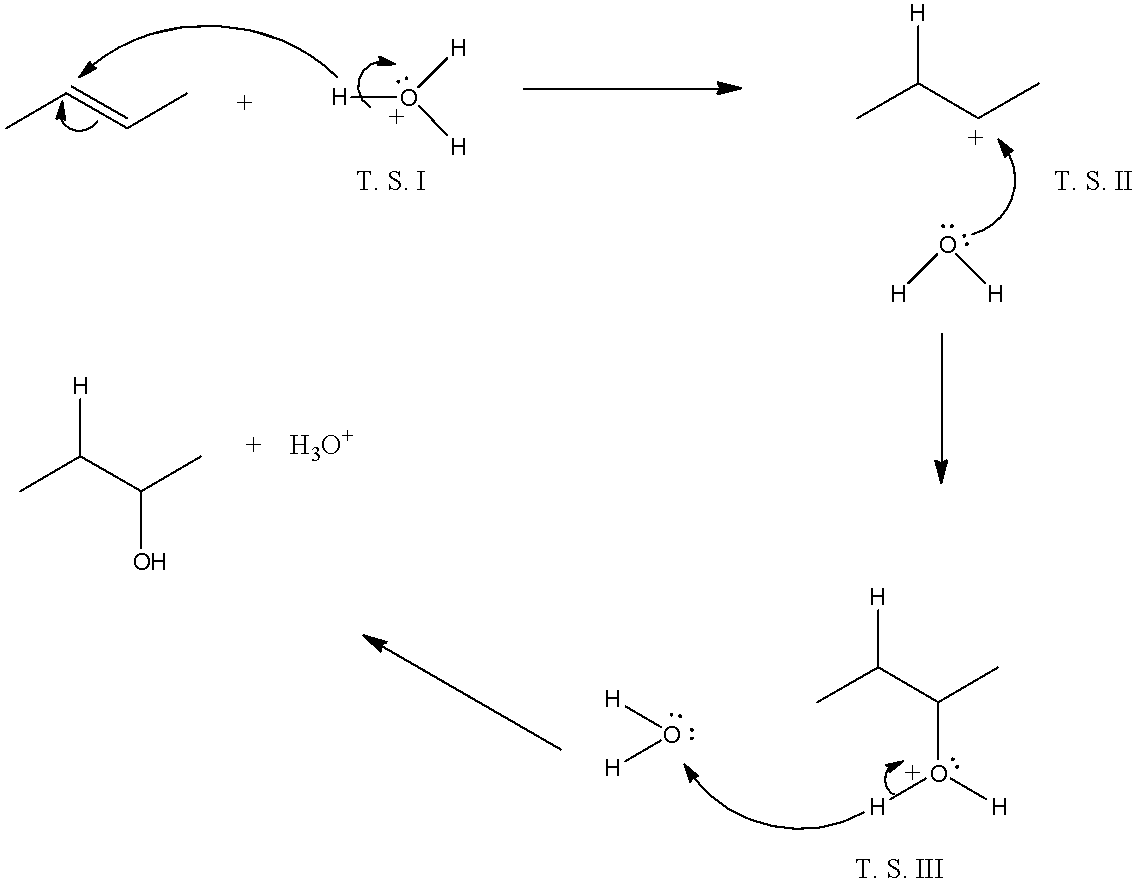
So, there are three transition states.
Note:
When symmetrical alkene is treated with water, then only one alcohol is formed, but when the alkene is unsymmetrical then the hydroxyl ion will be attached to the carbon atom having a lesser number of hydrogen atoms.
Complete answer:
When the alkene is hydrated or treated with water in the presence of an acid, then there is the formation of alcohol, in this reaction, there are three steps, i.e., the addition of a proton to the alkene from hydronium ion, water molecule attacks the carbocation, and removal of a hydrogen ion from the oxonium ion.
All the steps given above have a transition state.
The hydration of alkene involves strong acids like hydrochloric acid (HCl), sulfuric acid (${{H}_{2}}S{{O}_{4}}$), etc. The proton from reacts with water to form hydronium ion (${{H}_{3}}{{O}^{+}}$), as shown below:
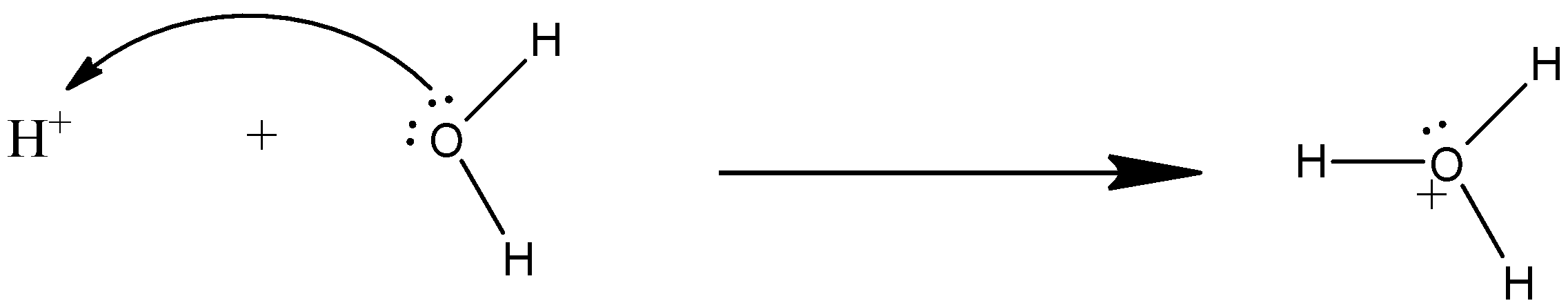
The mechanism takes place as, first, in the alkene the double breaks, and at one carbon atom there will be the addition of hydrogen ion from the hydronium ion and the other carbon atom will have a positive charge. Now, at this positive charge, the water molecule will attack. This will create a positive charge on the oxygen atom, so another water molecule will take a hydrogen ion from the oxonium ion. In all three steps transition states are formed and alcohol is formed. The reactions are given below:

So, there are three transition states.
Note:
When symmetrical alkene is treated with water, then only one alcohol is formed, but when the alkene is unsymmetrical then the hydroxyl ion will be attached to the carbon atom having a lesser number of hydrogen atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




