
What is the total number of $\sigma $and $\pi $ bonds in pyrophosphoric acid?
(A) $8,2$
(B) $10,2$
(C) $12,2$
(D) $8,4$
Answer
569.4k+ views
Hint: As we know that phosphorus forms a number of oxoacids and the condensation of two molecules of phosphoric acid $({H_3}P{O_4})$ results in the formation of pyrophosphoric acid with a chemical formula $({H_4}{P_2}{O_7})$and sigma and pi bonds are formed by overlapping of atomic orbitals.
Complete Step by step answer: We know that phosphorus on oxidation and condensation forms various oxoacids and the condensation of two molecules of phosphoric acid results in the pyrophosphoric acid formation that has a formula of $({H_4}{P_2}{O_7})$.
We also know that $\sigma $and $\pi $bonds are formed by overlapping atomic orbitals. End to end overlapping of atomic orbitals results in sigma bonds formation and pi bond is formed by sideways overlapping of atomic orbitals.
Now let us first see the structure of pyrophosphoric acid to make it clear:
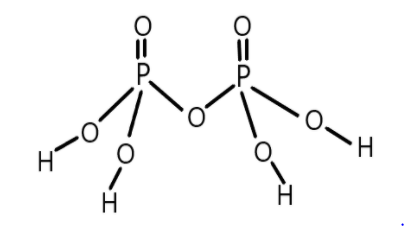
As we can see in the structure that it contains four $P - OH$ bonds, two $P = O$ bonds and one $P - O - P$ bond and we know that sigma bonds are the result of head to head overlapping of orbitals thus it generally forms a single bond. Therefore we can say that all the single bonds present in pyrophosphoric acid are sigma bonds. So, a total of 10 single bonds are there between oxygen and hydrogen and phosphorus and oxygen.
Now talking about the double bonds, we know that they are formed by sideways overlapping of orbitals and their axes are parallel to each other resulting in double bond which generally exist in combination with a sigma bond thus one of the double bond is sigma and the other is pi. Therefore, in pyrophosphoric acid there are a total two pi bonds between phosphorus and oxygen and two more sigma bonds make it a total of twelve sigma bonds.
Therefore the correct answer is (C) i.e. $12,2$.
Note: Always remember that a sigma bond corresponds to a single bond and a double bond corresponds to one sigma and one pi-bond whereas a triple bond generally involves one sigma and two pi-bonds.
Complete Step by step answer: We know that phosphorus on oxidation and condensation forms various oxoacids and the condensation of two molecules of phosphoric acid results in the pyrophosphoric acid formation that has a formula of $({H_4}{P_2}{O_7})$.
We also know that $\sigma $and $\pi $bonds are formed by overlapping atomic orbitals. End to end overlapping of atomic orbitals results in sigma bonds formation and pi bond is formed by sideways overlapping of atomic orbitals.
Now let us first see the structure of pyrophosphoric acid to make it clear:

As we can see in the structure that it contains four $P - OH$ bonds, two $P = O$ bonds and one $P - O - P$ bond and we know that sigma bonds are the result of head to head overlapping of orbitals thus it generally forms a single bond. Therefore we can say that all the single bonds present in pyrophosphoric acid are sigma bonds. So, a total of 10 single bonds are there between oxygen and hydrogen and phosphorus and oxygen.
Now talking about the double bonds, we know that they are formed by sideways overlapping of orbitals and their axes are parallel to each other resulting in double bond which generally exist in combination with a sigma bond thus one of the double bond is sigma and the other is pi. Therefore, in pyrophosphoric acid there are a total two pi bonds between phosphorus and oxygen and two more sigma bonds make it a total of twelve sigma bonds.
Therefore the correct answer is (C) i.e. $12,2$.
Note: Always remember that a sigma bond corresponds to a single bond and a double bond corresponds to one sigma and one pi-bond whereas a triple bond generally involves one sigma and two pi-bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




