
There is a large difference in the boiling points of butanal and butan-1-ol due to:
A) Intermolecular hydrogen bonding in butan-1-ol.
B) Intramolecular hydrogen bonding in butanal.
C) Higher molecular mass of butan-1-ol.
D) Resonance shown by butanal.
Answer
566.4k+ views
Hint: The answer to the question here is dependent on the fact about the variation in boiling points based on the facts about polarity and also the dipole – dipole interaction and solubility and also the hydrogen bonding.
Complete Solution :
In our previous chapters of chemistry, we have come across the various bonding and the interaction that exists between them in the same molecule and also different molecules.
Let us now see the reasons or the factors that affect the boiling points.
Boiling point of chemicals depends on various factors among which hydrogen bonding also plays a main role.
- Let us take butanal which is the name given for the aldehyde functional group and the butanal structure is as shown:
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-CHO\]
Here, the carbonyl group of this aldehyde has weak dipole – dipole interaction between them and thus this compound is difficult to be soluble in aqueous solution like water because the hydrogen do not dissociate easily.
- In case of butan-1-ol, the structure having alcohol functional group is shown below:
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}-OH\]
This compound has an alcohol group that is polar in nature and thus they are easily soluble in water.
The other factor is that the intermolecular hydrogen bonding exists in butan-1-ol with other same or different alcohol due its polarity as it can dissociate hydrogen easily. The bonding is shown below:
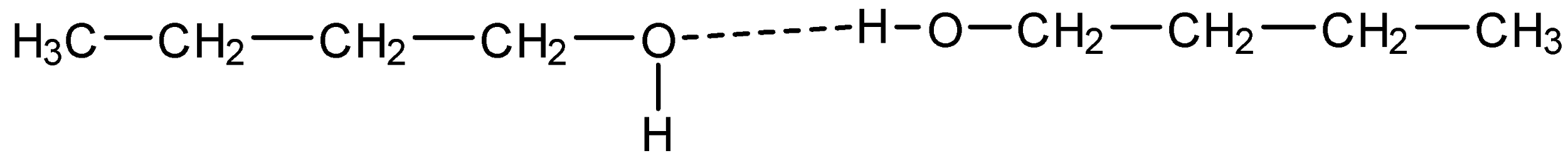
And because of this bonding the boiling point is low for butan-1-ol and high for butanal.
Thus, there is a huge difference in boiling points of two molecules.
So, the correct answer is “Option A”.
Note: Note that intermolecular and intramolecular bonding are the different terminologies given for the type of bonding where intermolecular bonding refers to the bonding between two atoms of same or different molecules whereas intramolecular bonding is the bonding interaction of atoms of the same molecule within itself.
Complete Solution :
In our previous chapters of chemistry, we have come across the various bonding and the interaction that exists between them in the same molecule and also different molecules.
Let us now see the reasons or the factors that affect the boiling points.
Boiling point of chemicals depends on various factors among which hydrogen bonding also plays a main role.
- Let us take butanal which is the name given for the aldehyde functional group and the butanal structure is as shown:
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-CHO\]
Here, the carbonyl group of this aldehyde has weak dipole – dipole interaction between them and thus this compound is difficult to be soluble in aqueous solution like water because the hydrogen do not dissociate easily.
- In case of butan-1-ol, the structure having alcohol functional group is shown below:
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}-OH\]
This compound has an alcohol group that is polar in nature and thus they are easily soluble in water.
The other factor is that the intermolecular hydrogen bonding exists in butan-1-ol with other same or different alcohol due its polarity as it can dissociate hydrogen easily. The bonding is shown below:
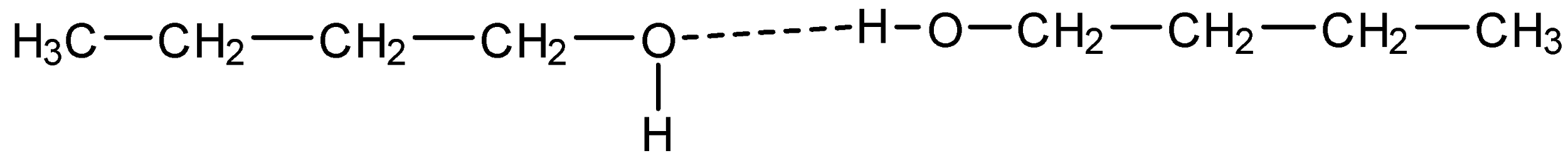
And because of this bonding the boiling point is low for butan-1-ol and high for butanal.
Thus, there is a huge difference in boiling points of two molecules.
So, the correct answer is “Option A”.
Note: Note that intermolecular and intramolecular bonding are the different terminologies given for the type of bonding where intermolecular bonding refers to the bonding between two atoms of same or different molecules whereas intramolecular bonding is the bonding interaction of atoms of the same molecule within itself.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




