
The wrong statement about ${{\text{N}}_2}{\text{O}}$ is that:
A. It is nitrous oxide.
B. It is least reactive oxide of nitrogen
C. It is not a linear molecule
D. It is known as laughing gas
Answer
572.1k+ views
Hint:${{\text{N}}_2}{\text{O}}$ is known as dinitrogen oxide. The oxidation state of nitrogen is $ + 1$. It is a stable oxide. Dinitrogen oxide shows $sp$ hybridization. It has an exhilarating effect on inhalation.
Complete answer:
Nitrogen forms various oxides such as nitrogen oxide, nitrogen dioxide, dinitrogen oxide, and dinitrogen trioxide and dinitrogen pentoxide.
The oxidation state of nitrogen in dinitrogen oxide is as follows:
The oxidation state of an oxygen atom is $ - 2$.
\[\left( {x\, \times 2} \right) + \left( { - 2\, \times 1} \right)\]
\[x = + 1\]
So, the oxidation state of nitrogen in dinitrogen oxide is \[ + 1\].
The nitrogen in \[ + 1\] oxidation state is known as nitrous. So, it is correct that dinitrogen oxide is nitrous oxide.
Octet of each atom of nitrous oxide is complete, so it is stable. Stability is indirectly proportional to the reactivity. Thus it is true that nitrous oxide is the least reactive oxide of nitrogen.
The structure of the nitrous oxide is as follows:
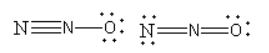
Nitrous oxide is a linear molecule, so the statement that it is not a linear molecule is not true.
The nitrous oxide on inhalation causes an intoxicating effect, so it is true that it is known as laughing gas.
Therefore option (C) It is not a linear molecule, is correct.
Note:
Nitrous oxide is used as anaesthesia in surgery. It has an analgesic effect. The hybridization of the nitrogen atom in nitrous oxide is sp. Nitrous oxide is diamagnetic. It is a colourless gas. It is neutral to a litmus test.
Complete answer:
Nitrogen forms various oxides such as nitrogen oxide, nitrogen dioxide, dinitrogen oxide, and dinitrogen trioxide and dinitrogen pentoxide.
The oxidation state of nitrogen in dinitrogen oxide is as follows:
The oxidation state of an oxygen atom is $ - 2$.
\[\left( {x\, \times 2} \right) + \left( { - 2\, \times 1} \right)\]
\[x = + 1\]
So, the oxidation state of nitrogen in dinitrogen oxide is \[ + 1\].
The nitrogen in \[ + 1\] oxidation state is known as nitrous. So, it is correct that dinitrogen oxide is nitrous oxide.
Octet of each atom of nitrous oxide is complete, so it is stable. Stability is indirectly proportional to the reactivity. Thus it is true that nitrous oxide is the least reactive oxide of nitrogen.
The structure of the nitrous oxide is as follows:
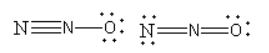
Nitrous oxide is a linear molecule, so the statement that it is not a linear molecule is not true.
The nitrous oxide on inhalation causes an intoxicating effect, so it is true that it is known as laughing gas.
Therefore option (C) It is not a linear molecule, is correct.
Note:
Nitrous oxide is used as anaesthesia in surgery. It has an analgesic effect. The hybridization of the nitrogen atom in nitrous oxide is sp. Nitrous oxide is diamagnetic. It is a colourless gas. It is neutral to a litmus test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




