
The type of magnetism exhibited by ${{\left[ Mn{{\left( {{H}_{2}}O \right)}_{6}} \right]}^{2+}}$ ion is:
A. paramagnetism
B. diamagnetism
C. both a and b
D. none of these
Answer
577.2k+ views
Hint: To find the type of magnetism, we need the number of unpaired electrons of the central atom of the compound. So, always ensure that we are counting the unpaired electrons of the given atom or ion only while finding it and not all the valence electrons.
Complete step by step solution:
-There can enter 2 electrons in opposite orientation in 1 orbital. Individual electron spins are added to get a total spin and individual angular momenta are added to give total angular momentum.
-Based on magnetic behaviour, atoms are classified as paramagnetic and diamagnetic. Diamagnetic atoms or ions have all paired electrons in their shell while paramagnetic atoms or ions have 1 or more unpaired electrons.
-The given compound is a coordination compound. The magnetic properties of such compounds depend on the central atom of the compounds. The central atom here in this compound is Mn. The ligand present here is water which is neutral and so the charge of the ion is the charge of the central atom itself.
-The atomic number of Mn is 25. Now we see that 2 electrons have been removed in Mn atom to form a cation with charge +2. We know that the electrons are removed from the outermost shell. So we need to see the electronic configuration of Mn atom first which is
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{5}}$
The outermost shell is 4s. So, when 2 electrons are removed, the configuration will become $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}$ .
-We know that the d subshell has 5 orbitals and electrons are filled first as unpaired and then are paired. So 5 electrons will occupy 5 different orbitals of the subshell. It can be shown as
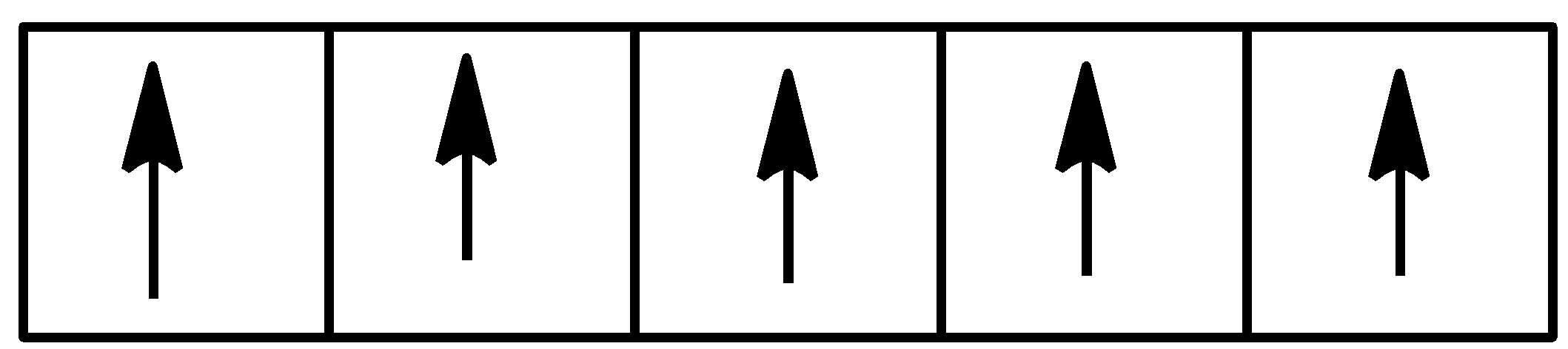
So we can see that there are 5 unpaired electrons.
-Since there are unpaired electrons present in the central atom, we can say that the given ion is paramagnetic. Not only it is paramagnetic but it has the maximum value of magnetic moment which is found by the formula
$\mu =\sqrt{n\left( n+2 \right)}$
Therefore the correct option is A.
Note: The charge of the ion is not always the charge of the central atom. It is only in the case of the neutral ligands. For other compounds, we find the charge on the central atom by the concept that the sum of all the charges of the atoms on the ion is equal to the charge present on that compound.
Complete step by step solution:
-There can enter 2 electrons in opposite orientation in 1 orbital. Individual electron spins are added to get a total spin and individual angular momenta are added to give total angular momentum.
-Based on magnetic behaviour, atoms are classified as paramagnetic and diamagnetic. Diamagnetic atoms or ions have all paired electrons in their shell while paramagnetic atoms or ions have 1 or more unpaired electrons.
-The given compound is a coordination compound. The magnetic properties of such compounds depend on the central atom of the compounds. The central atom here in this compound is Mn. The ligand present here is water which is neutral and so the charge of the ion is the charge of the central atom itself.
-The atomic number of Mn is 25. Now we see that 2 electrons have been removed in Mn atom to form a cation with charge +2. We know that the electrons are removed from the outermost shell. So we need to see the electronic configuration of Mn atom first which is
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{5}}$
The outermost shell is 4s. So, when 2 electrons are removed, the configuration will become $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}$ .
-We know that the d subshell has 5 orbitals and electrons are filled first as unpaired and then are paired. So 5 electrons will occupy 5 different orbitals of the subshell. It can be shown as

So we can see that there are 5 unpaired electrons.
-Since there are unpaired electrons present in the central atom, we can say that the given ion is paramagnetic. Not only it is paramagnetic but it has the maximum value of magnetic moment which is found by the formula
$\mu =\sqrt{n\left( n+2 \right)}$
Therefore the correct option is A.
Note: The charge of the ion is not always the charge of the central atom. It is only in the case of the neutral ligands. For other compounds, we find the charge on the central atom by the concept that the sum of all the charges of the atoms on the ion is equal to the charge present on that compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




